Abstract
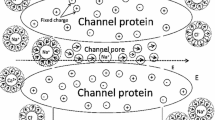
Quantum chemical model calculations were carried out for modeling the ion transport through an isolated ion channel of a cell membrane. An isolated part of a natural ion channel was modeled. The model channel was a calixarene derivative, hydrated sodium and potassium ions were the models of the transported ion. The electrostatic potential of the channel and the energy of the channel-ion system were calculated as a function of the alkali ion position. Both attractive and repulsive ion-channel interactions were found. The calculations – namely the dependence of the system energy and the atomic charges of the water molecules with respect to the position of the alkali ion in the channel – revealed the molecular-structural background of the potassium selectivity of this artificial ion channel. It was concluded that the studied ion channel mimics real biological ion channel quite well.

Transportation behavior of alkali ions through a cell membrane ion channel










Similar content being viewed by others
References
Hille B (2001) Ion channels of excitable membranes, 3rd edn. Sinauer Associates, Sunderland, MA, USA
Aidley DJ, Stanfield PR (1996) Ion channels: molecules in action. Cambridge University Press, Cambridge, UK
Kew JNC (2010) Davies CH (2010) Ion channels: from structure to function. Oxford University Press, Oxford, UK
Gillespie D, Eisenberg RS (2002) Physical descriptions of experimental selectivity measurements in ion channels. Eur Biophys J 31:454–466. doi:10.1007/s00249-002-0239-x
Hall LT, Hill CD, Cole JH, Städler B, Caruso F, Mulvaney P, Wrachtrup J, Hollenberg LCL (2010) Monitoring ion-channel function in real time through quantum decoherence. PNAS 107:18777–18782. doi:10.1073/pnas.1002562107
Siwy Z, Ausloos M, Ivanova K (2002) Correlation studies of open and closed state fluctuations in an ion channel: analysis of ion current through a large-conductance locust potassium channel. Phys Rev E 65:031907. doi:10.1103/PhysRevE.65.031907
Guidoni L, Carloni P (2002) Potassium permeation through the KcsA channel: a density functional study. Biochim Biophys Acta 1563:1–6. doi:10.1016/j.bpc.2006.04.008
Roux B (2002) Computational studies of the Gramicidin channel. Acc Chem Res 35:366–375. doi:10.1021/ar010028v
Grigorenko BL, Nemukhin AV, Topol IA, Burt SK (2002) Modeling of Biomolecular Systems with the Quantum Mechanical and Molecular Mechanical Method Based on the Effective Fragment Potential Technique: Proposal of Flexible Fragments. J Phys Chem A 106:10663–10672. doi:10.1021/jp026464w
Vogel C (2002) Modeling transport across biological membranes. Structural Studies Division MRC Laboratory of Molecular Biology, Hills Road, Cambridge. http://polaris.icmb.utexas.edu/people/cvogel/Dogs/mini1_gapjct.doc
Liu JL (2011) A quantum corrected Poisson-Nernst-Planck model for biological ion channels. www.nhcue.edu.tw/∼jinnliu/proj/Device/2011QCPNP.pdf
Abad E, Reingruber J, Sansom MSP (2009) On a novel rate theory for transport in narrow ion channels and its application to the study of flux optimization via geometric effects. J Chem Phys 130(055101):1–18. doi:10.1063/1.3077205
Poznanski RR, Bell J (2000) A dendritic cable model for the amplification of synaptic potentials by an ensemble average of persistent sodium channels. Math Biosci 166:101–121. doi:10.1016/S0025-5564(00)00031-6
Poznanski RR, Bell J (2000) Theoretical analysis of the amplification of synaptic potentials by small clusters of persistent sodium channels in dendrites. Math Biosci 166:123–147. doi:10.1016/S0025-5564(00)00032-8
Chen JL, Tsai CH, Chen YY (1995) The application of hidden markov model to the problem of ion channel modeling; National Science Council, NSC 84-2213-E-216-020
Ash WL, Zlomislic MR, Oloo EO, Tieleman DP (2004) Computer simulations of membrane proteins. Biochim Biophys Acta 1666:158–189. doi:10.1016/j.bbamem.2004.04.012
Tieleman DP, Biggin PC, Smith GR, Sansom MSP (2001) Simulation approaches to ion channel structure–function relationships. Q Rev Biophys 34:473–561. doi:10.1017/S0033583501003729
Bohmer V (1995) Calixarenes, macrocycles with (almost) unlimited possibilities. Angew Chem Int Ed Engl 34:713–745. doi:10.1002/anie.199507131
Tanaka Y, Kobuke Y, Sokabe M (1995) A non-peptidic ion channel with K+ selectivity. Angew Chem Int Ed 34:693–694. doi:10.1002/anie.199506931
Chen WH, Nishikawa M, Tan SD, Yamamura M, Satake A, Kobuke Y (2004) Tetracyanoresorcin[4]arene ion channel shows pH dependent conductivity change. Chem Commun (Camb) 7:872–873. doi:10.1039/B312952G
Kulikov OV, Li R, Gokel GW (2009) A synthetic ion channel derived from a metallogallarene capsule that functions in phospholipid bilayers. Angew Chem Int Ed Engl 48:375–377. doi:10.1002/anie.200804099
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA Jr, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi MR, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (1998) Gaussian 98, Revision A.3. Gaussian Inc, Pittsburgh, PA
Billes F, Mohammed-Ziegler I (2002) Ab initio equilibrium geometry and vibrational spectroscopic study of 25,26,27,28-tetrahydroxycalix[4]arene. Supramol Chem 14:451–459. doi:10.1080/10610270212491
Furer VL, Borisoglebskaya EI, Kovalenko VI (2005) Band intensity in the IR spectra and conformations of calix[4]arene and thiacalix[4]arene. Spectrochim Acta A 61:355–359. doi:10.1016/j.saa.2004.05.009
Backes AC, Schatz J, Siehl HU (2002) GIAO-DFT calculated and experimentally derived complexation-induced chemical shifts of calix[4]arene–solvent inclusion complexes. J Chem Soc -Perkin Trans 2:484–488. doi:10.1039/B110078P
Choe JI, Lee SH (2004) Ab Initio Study of the conformational isomers of tetraethyl and triethyl esters of calix[4]arene. B Kor Chem Soc 25:553–556. doi:10.5012/bkcs.2004.25.6.847
Mäkinen M, Jalkanen JP, Vainiotalo P (2002) Conformational properties and intramolecular hydrogen bonding of tetraethyl resorcarene: an ab initio study. Tetrahedron 58:8591–8596. doi:10.1016/S0040-4020(02)00863-3
Rempe SB, Pratt LR (2000). Ion hydration studies aimed at ion channel selectivity. Theor Chem Mol Phys T-12. Los Alamos. http://www.t12.lanl.gov/projects/rempe.pratt.00.pdf
Acknowledgments
The authors would like to express their gratitude for the help of Prof. Reiner Saltzer (Institute of Analytical Chemistry, Dresden University of Technology). I. M-Z participated in this work as a former staff member of IR and Raman Laboratory, Chemical Research Center of the Hungarian Academy of Sciences, Budapest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Billes, F., Mohammed-Ziegler, I. & Mikosch, H. Transportation behavior of alkali ions through a cell membrane ion channel. A quantum chemical description of a simplified isolated model. J Mol Model 18, 3627–3637 (2012). https://doi.org/10.1007/s00894-012-1364-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1364-9