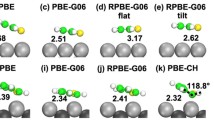
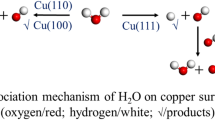
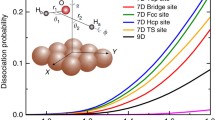
Abstract
A first-principles theoretical study of the water-Cu(111) interface based on density functional calculations is reported. Using differently sized surface models: p(2 × 2), p(4 × 4) and p(4 × 5), we found out that the adsorption energy of a H2O monomer does not significantly change with the surface model though the adsorption geometry is sensitive to the choice of the super-cell surface and, also, to the coverage. Molecular dynamics simulations on the Born-Oppenheimer surface of liquid water on a Cu(111) surface reveal that H2O in the first solvent layer adsorbs O-down and that the H-bond network is weaker upon adsorption on the Cu. Furthermore, absolute electrochemical potentials are presented and compared to the potential of zero charge obtained experimentally and theoretically.








Similar content being viewed by others
References
Hodgson A, Haq S (2009) Water adsorption and the wetting of metal surfaces. Surf Sci Rep 64:381–451. doi:10.1016/j.surfrep.2009.07.001
Henderson M (2002) The interaction of water with solid surfaces: Fundamental aspects revisited. Surf Sci Rep 46:1–308. doi:10.1016/S0167-5729(01)00020-6
Thiel P, Madey T (1987) The interaction of water with solid surfaces: Fundamental aspects. Surf Sci Rep 7:211–385. doi:10.1016/0167-5729(87)90001-X
Morgenstern K, Nieminen J (2002) Intermolecular bond length of ice on Ag(111). Phys Rev Lett 88:066102. doi:10.1103/PhysRevLett.88.066102
Morgenstern K, Rieder K (2002) Formation of the cyclic ice hexamer via excitation of vibrational molecular modes by the scanning tunneling microscope. J Chem Phys 116:5746–5753. doi:10.1063/1.1453965
Cerdá J, Michaelides A, Bocquet M, Feibelman P, Mitsui T, Rose M, Fomin E, Salmeron M (2010) Novel water overlayer growth on Pd(111) characterized with scanning tunneling microscopy and density functional theory. Phys Rev Lett 93:116101. doi:10.1103/PhysRevLett.93.116101
Nie S, Feibelman P, Bartelt N, Thürmer K (2010) Pentagons and heptagons in the first water layer on Pt(111). Phys Rev Lett 105:026102. doi:10.1103/PhysRevLett.105.026102
Carrasco J, Michaelides A, Forster M, Haq S, Raval R, Hodgson A (2009) A one-dimensional ice structure built from pentagons. Nat Mater 8:427–431. doi:10.1038/nmat2403
Michaelides A, Ranea V, De Andres P, King D (2003) General model for water monomer adsorption on close-packed transition and noble metal surfaces. Phys Rev Lett 90:216102. doi:10.1103/PhysRevLett.90.216102
Tang Q, Chen Z (2007) Density functional slab model studies of water adsorption on flat and stepped Cu surfaces. Surf Sci 601:954–964. doi:10.1016/j.susc.2006.11.036
Wang G, Nakamura J (2010) Structure sensitivity for forward and reverse water-gas shift reactions on copper surfaces: a DFT study. J Phys Chem Lett 1:3053–3057. doi:10.1021/jz101150w
Fajín J, Illas F, Gomes J (2009) Effect of the exchange-correlation potential and of surface relaxation on the description of the H2O dissociation on Cu(111). J Chem Phys 130:224702–224710. doi:10.1063/1.3149851
Gokhale A, Dumesic J, Mavrikakis M (2008) On the mechanism of low-temperature water gas shift reaction on copper. J Am Chem Soc 130:1402–1414. doi:10.1021/ja0768237
Feibelman P (2002) Partial dissociation of water on Ru(0001). Science 295:99–102. doi:10.1126/science.1065483
Vassilev P, van Santen R, Koper M (2005) Ab initio studies of a water layer at transition metal surfaces. J Chem Phys 122:054701–054713. doi:10.1063/1.1834489
Poissier A, Ganeshan S, Fernández-Serra M (2011) The role of hydrogen bonding in water–metal interactions. Phys Chem Chem Phys 13:3375–3384. doi:10.1039/C0CP00994F
Dion M, Rydberg H, Schröder E, Langreth D, Lundqvist B (2004) Van der Waals density functional for general geometries. Phys Rev Lett 92:246401–246405. doi:10.1103/PhysRevLett.92.246401
Carrasco J, Santra B, Klimeš J, Michaelides A (2011) To wet or not to wet? Dispersion forces tip the balance for water ice on metals. Phys Rev Lett 106:026101–026105. doi:10.1103/PhysRevLett.106.026101
Toney M, Howard J, Richer J, Borges G, Gordon J, Melroy O, Wiesler D, Yee D, Sorensen L (1994) Voltage-dependent ordering of water molecules at an electrode–electrolyte interface. Nature 368:444–446. doi:10.1038/368444a0
Ataka K, Yotsuyanagi T, Osawa M (1996) Potential-dependent reorientation of water molecules at an electrode/electrolyte interface studied by surface-enhanced infrared absorption spectroscopy. J Phys Chem 100:10664
Price D, Halley J (1995) Molecular dynamics, density functional theory of the metal–electrolyte interface. J Chem Phys 102:6603–6613. doi:10.1063/1.469376
Izvekov S, Mazzolo A, VanOpdorp K, Voth G (2001) Ab initio molecular dynamics simulation of the Cu(110)–water interface. J Chem Phys 114:3248–3258. doi:10.1063/1.1342859
Izvekov S, Voth G (2001) Ab initio molecular dynamics simulation of the Ag(111)-water interface. J Chem Phys 115:7196–7207. doi:10.1063/1.1403438
Schnur S, Gross A (2009) Properties of metal–water interfaces studied from first principles. New J Phys 11:125003. doi:10.1088/1367-2630/11/12/125003
Zelsmann H (1995) Temperature dependence of the optical constants for liquid H2O and D2O in the far IR region. J Mol Struct 350:95–114. doi:10.1016/0022-2860(94)08471-S
Lock A, Bakker H (2002) Temperature dependence of vibrational relaxation in liquid H2O. J Chem Phys 117:1708–1714. doi:10.1063/1.1485966
Tsiplakides D, Archonta D, Vayenas C (2007) Absolute potential measurements in solid and aqueous electrochemistry using two Kelvin probes and their implications for the electrochemical promotion of catalysis. Top Catal 44:469–479. doi:10.1007/s11244-006-0139-x
Taylor C, Wasileski S, Filhol J, Neurock M (2006) First principles reaction modeling of the electrochemical interface: Consideration and calculation of a tunable surface potential from atomic and electronic structure. Phys Rev B 73:165402. doi:10.1103/PhysRevB.73.165402
Taylor C, Kelly R, Neurock M (2006) First Principles Modeling of Structure and Reactivity at the Metal/Water Interface. Dissertation, University of Virginia
McGrath M, Siepmann J, Kuo I, Mundy C (2006) Vapor-liquid equilibria of water from first principles: comparison of density functionals and basis sets. Mol Phys 104:3619–3626. doi:10.1080/00268970601014781
Liu L, Krack M, Michaelides A (2009) Interfacial water: A first principles molecular dynamics study of a nanoscale water film on salt. J Chem Phys 130:234702–234714. doi:10.1063/1.3152845
Nosé S (1984) A unified formulation of the constant temperature molecular dynamics methods. J Chem Phys 81:511–520. doi:10.1063/1.447334
Nosé S (1984) A molecular dynamics method for simulations in the canonical ensemble. Mol Phys 52:255–268. doi:10.1080/00268978400101201
Zhang X, Liu Q, Zhu A (2007) An improved fully flexible fixed-point charges model for water from ambient to supercritical condition. Fluid Phase Equilib 262:210–216. doi:10.1016/j.fluid.2007.09.005
Heinz H, Vaia R, Farmer B, Naik R (2008) Accurate simulation of surfaces and interfaces of face-centered cubic metals using 12–6 and 9–6 Lennard-Jones potentials. J Phys Chem C 112:17281–17290. doi:10.1021/jp801931d
CP2K Developers Home Page http://cp2k.berlios.de.
Hansen J, McDonald I (1986) Theory of simple liquids, 2nd edn. Academic, New York
Trasatti S (1986) The absolute electrode potential: an explanatory note. Pure Appl Chem 58:955–966. doi:10.1351/pac198658070955S
Kresse G, Hafner J (1993) Ab initio molecular dynamics for liquid metals. Phys Rev B 47:558–561. doi:10.1103/PhysRevB.47.558
Kresse G, Furthmüller J (1996) Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater Sci 6:15–50. doi:10.1016/0927-0256(96)00008-0
Kresse G, Furthmüller J (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B 54:11169–11186. doi:10.1103/PhysRevB.54.11169
Blöchl P (1994) Projector augmented-wave method. Phys Rev B 50:17953–17979. doi:10.1103/PhysRevB.50.17953
Kresse G, Joubert D (1999) From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B 59:1758–1775. doi:10.1103/PhysRevB.59.1758
Perdew J, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868. doi:10.1103/PhysRevLett.77.3865
Otani M, Hamada I, Sugino O, Morikawa Y, Okamoto Y, Ikeshoji T (2008) Structure of the water/platinum interface––a first principles simulation under bias potential. Phys Chem Chem Phys 10:3609–3612. doi:10.1039/B803541E
Miura N, Yamada H, Moon A (2010) Intermolecular vibrational study in liquid water and ice by using far infrared spectroscopy with synchrotron radiation of MIRRORCLE 20. Spectrochimica Acta Part A 77:1048–1053. doi:10.1016/j.saa.2010.08.071
Clavilier J, Huong C (1969) Compt Rend Ser C 269:736
Walbran S, Mazzolo A, Halley J, Price D (1998) Model for the electrostatic response of the copper–water interface. J Chem Phys 109:8076–8081. doi:10.1063/1.477455
Acknowledgments
This work was funded by the Spanish Ministerio de Ciencia e Innovación, MICINN, projects MAT2008-4918 and CSD2008-0023. RN thanks the Junta de Andalucía for a pre-doctoral grant (P08-FQM-3661). Part of the calculations has been carried out at the Barcelona Supercomputing Center -Centro Nacional de Supercomputación (Spain).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nadler, R., Sanz, J.F. First-principles molecular dynamics simulations of the H2O / Cu(111) interface. J Mol Model 18, 2433–2442 (2012). https://doi.org/10.1007/s00894-011-1260-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-011-1260-8