Abstract
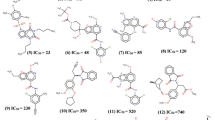
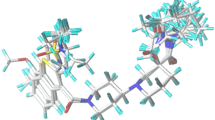
Presently, an in silico modeling was carried out on a series of 63 phosphonic acid-containing thiazole derivatives as fructose-1,6-bisphosphatase (FBPase) inhibitors using CoMFA/CoMSIA and molecular docking methods. The CoMFA and CoMSIA models using 51 molecules in the training set gave r 2cv values of 0.675 and 0.619, r 2 values of 0.985 and 0.979, respectively. The systemic external validation indicated that our CoMFA and CoMSIA models possessed high predictive powers with r 20 values of 0.995 and 0.994, r 2m(test) values of 0.887 and 0.860, respectively. The 3D contour maps of the CoMFA and CoMSIA provided smooth and interpretable explanation of the structure-activity relationship for the inhibitors. Molecular docking studies revealed that a phosphonic group was essential for binding to the AMP binding site, and some key features were also identified. The analyses of the 3D contour plots and molecular docking results permitted interesting conclusions about the effects of different substituent groups at different positions of the common scaffold, which might guide the design of novel FBPase inhibitors with higher activity and bioavailability. A set of 60 new analogues were designed by utilizing the results revealed in the present study, and were predicted with significantly improved potencies in the developed models. The findings can be quite useful to aid the designing of new fructose-1,6-biphophatase inhibitors with improved biological response.

An in silico modeling was carried out on a series of 63 phosphonic acid-containing thiazole derivatives as fructose-1,6-bisphosphatase (FBPase) inhibitors using CoMFA/CoMSIA and molecular docking methods. The findings can be quite useful to aid the designing of new fructose-1,6-biphophatase inhibitors with improved biological response









Similar content being viewed by others
References
Heng S, Harris KM, Kantrowitz ER (2010) Designing inhibitors against fructose 1,6-bisphosphatase: Exploring natural products for novel inhibitor scaffolds. Eur J Med Chem 45:1478–1484
Kitas E, Mohr P, Kuhn B, Hebeisen P, Wessel HP, Haap W, Ruf A, Benz J, Joseph C, Huber W, Sanchez RA, Paehler A, Benardeau A, Gubler M, Schott B, Tozzo E (2010) Sulfonylureido thiazoles as fructose-1,6-bisphosphatase inhibitors for the treatment of Type-2 diabetes. Bioorg Med Chem Lett 20:594–599
Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI (1992) Increased rate of gluconeogenesis in type- II diabetes-mellitus-A C-13 nuclear-magnetic-resonance study. J Clin Invest 90:1323–1327
Tsukada T, Tamaki K, Tanaka J, Takagi T, Yoshida T, Okuno A, Shiiki T, Takahashi M, Nishi T (2010) A prodrug approach towards the development of tricyclic-based FBPase inhibitors. Bioorg Med Chem Lett 20:2938–2941
Hebeisen P, Kuhn B, Kohler P, Gubler M, Huber W, Kitas E, Schott B, Benz J, Joseph C, Ruf A (2008) Allosteric FBPase inhibitors gain 105 times in potency when simultaneously binding two neighboring AMP sites. Bioorg Med Chem Lett 18:4708–4712
Kebede M, Favaloro J, Gunton JE, Laybutt R, Shaw M, Wong N, Fam BC, Aston-Mourney K, Rantzau C, Zulli A, Proietto J, Andrikopoulos S (2008) Fructose-1,6-Bisphosphatase overexpression in pancreatic β-cells results in reduced insulin secretion. Diabetes 57:1887–1895
Mendicino J, Kratowich N, Oliver RM (1972) Role of enzyme-enzyme interactions in regulation of gluconeogenesis-properties and subunit structure of fructose 1,6-diphosphatase from swine kidney. J Biol Chem 247:6643–6650
Heng S, Gryncel KR, Kantrowitz ER (2009) A library of novel allosteric inhibitors against fructose 1,6-bisphosphatase. Bioorg Med Chem 17:3916–3922
Lan P, Xie MQ, Yao YM, Chen WN, Chen WM (2010) 3D-QSAR studies and molecular docking on [5-(4-amino-1 H-benzoimidazol-2-yl)-furan-2-yl]-phosphonic acid derivatives as fructose-1,6-biphophatase inhibitors. J Comput Aided Mol Des 24:993–1008
Dang Q, Kasibhatla SR, Xiao W, Liu Y, Dare J, Taplin F, Reddy KR, Scarlato GR, Gibson R, van Poelje PD, Potter SC, Erion MD (2010) Fructose-1,6-bisphosphatase inhibitors. 2. Design, synthesis, and structure-activity relationship of a series of phosphonic acid containing benzimidazoles that function as 5′-adenosinemonophosphate (AMP) mimics. J Med Chem 53:441–451
Dang Q, Brwon BS, Liu Y, Rydzewski RM, Robinson ED, van Poelje PD, Reddy RM, Erion MD (2009) Fructose-1,6-bisphosphatase inhibitors. 1. Purine phosphonic acids as novel AMP mimics. J Med Chem 52:2880–2898
Erion MD, Dang Q, Reddy MR, Kasibhatla SR, Huang J, Lipscomb WN, van Poelje PD (2007) Structure-guided design of AMP mimics that inhibit fructose-1,6-bisphosphatase with high affinity and specificity. J Am Chem Soc 129:15480–15490
Dang Q, Kasibhatla SR, Reddy KR, Jiang T, Reddy MR, Potter SC, Fujitaki JM, van Poelje PD, Huang J, Lipscomb WN, Erion MD (2007) Discovery of potent and specific fructose-1,6-bisphosphatase inhibitors and a series of orally-bioavailable phosphoramidase-sensitive prodrugs for the treatment of type 2 diabetes. J Am Chem Soc 129:15491–15502
Yamanaka G, Wilson T, Innaimo S, Bisacchi GS, Egli P, Rinehart JK, Zahler R, Colonno RJ (1999) Metabolic studies on BMS-200475, a new antiviral compound active against hepatitis B virus. Antimicrob Agents Chemother 43:190–193
Dang Q, Kasibhatla SR, Jiang T, Fan K, Liu Y, Taplin F, Schulz W, Cashion DK, Reddy KR, van Poelje PD, Fujitaki JM, Potter SC, Erion MD (2008) Discovery of phosphonic diamide prodrugs and their use for the oral delivery of a series of gructose 1,6-bisphosphatase inhibitors. J Med Chem 51:4331–4339
Dang Q, Liu Y, Cashion DK, Kasibhatla SR, Jiang T, Taplin F, Jacintho JD, Li H, Sun Z, Fan Y, DaRe J, Tian F, Li W, Gibson T, Lemus R, van Poelje PD, Potter SC, Erion MD (2011) Discovery of a series of phosphonic acid-containing thiazoles and orally bioavailable diamide prodrugs that lower glucose in diabetic animals through inhibition of fructose-1,6-bisphosphatase. J Med Chem 54:153–165
Cichero E, Cesarini S, Mosti L, Fossa P (2010) CoMFA and CoMSIA analyses on 4-oxo-1,4-dihydroquinoline and 4-oxo-1,4-dihydro-1,5-, -1,6- and −1,8-naphthyridine derivatives as selective CB2 receptor agonists. J Mol Model 16:677–691
Prado-Prado FJ, Uriarte E, Borges F, Gonzalez-Diaz H (2009) Multi-target spectral moments for QSAR and Complex Networks study of antibacterial drugs. Eur J Med Chem 44:4516–4521
Prado-Prado FJ, Ubeira FM, Borges F, Gonzalez-Diaz H (2009) Multiple distance and triadic census analysis of antiparasitic drugs complex networks. J Comput Chem 31:164–173
Trossini GHG, Guido RVC, Oliva G, Ferreira EI, Andricopulo AD (2009) Quantitative structure–activity relationships for a series of inhibitors of cruzain from Trypanosoma cruzi: Molecular modeling, CoMFA and CoMSIA studies. J Mol Graph Model 28:3–11
Lan P, Huang ZJ, Sun JR, Chen WM (2010) 3D-QSAR and molecular docking studies on fused pyrazoles as p38α mitogen-activated protein kinase inhibitors. Int J Mol Sci 11:3357–3374
Golbraikh A, Tropsha A (2002) Beware of q2. J Mol Graph Model 20:269–276
Roy PP, Roy K (2008) On some aspects of variable selection for partial least squares regression models. QSAR Comb Sci 27:302–313
Roy PP, Pau S, Mitra I, Roy K (2009) On two novel parameters for validation of predictive QSAR models. Molecules 14:1660–1701
SYBYL 8.1, Tripos Inc., 1699 South Hanley Rd., St. Louis, MO, 63144, USA
Lan P, Chen WN, Xiao GK, Sun PH, Chen WM (2010) 3D-QSAR and docking studies on pyrazolo[4,3-h]quinazoline-3-carboxamides as cyclin-dependent kinase 2 (CDK2) inhibitors. Bioorg Med Chem Lett 20:6764–6772
Khanfar MA, Youssef DTA, Sayed KAE (2010) 3D-QSAR studies of latrunculin-based actin polymerization inhibitors using CoMFA and CoMSIA approaches. Eur J Med Chem 45:3662–3668
Mouchlis VD, Mavromoustakos TM, Kokotos G (2010) Molecular docking and 3D-QSAR CoMFA studies on indole inhibitors of GIIA secreted phospholipase A2. J Chem Inf Model 50:1589–1601
Lan P, Chen WN, Chen WM (2011) Molecular modeling studies on imidazo[4,5-b]pyridine derivatives as Aurora A kinase inhibitors using 3D-QSAR and docking approaches. Eur J Med Chem 46:77–94
Pirhadi S, Ghasemi JB (2010) 3D-QSAR analysis of human immunodeficiency virus entry-1 inhibitors by CoMFA and CoMSIA. Eur J Med Chem 45:4897–4903
Acknowledgments
We gratefully acknowledge support for this research from the National Natural Science Foundation of China (Grant No. 81072554).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 79 kb)
Rights and permissions
About this article
Cite this article
Lan, P., Wu, ZW., Chen, WN. et al. Molecular modeling studies on phosphonic acid-containing thiazole derivatives: design for fructose-1,6-bisphosphatase inhibitors. J Mol Model 18, 973–990 (2012). https://doi.org/10.1007/s00894-011-1134-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-011-1134-0