Abstract
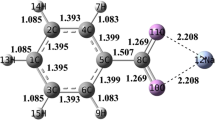
Different reaction pathways of the carboxylic group in a brown coal model were investigated by applying density function quantum chemical theory, examining the possible cross-linking and decomposition reactions between the hydrogen bonded carboxylic group–carboxylic group and the carboxylic group–hydroxyl group during the thermal pyrolysis process. The results show that bimolecular dehydration and decarboxylation of hydrogen bonded carboxylic groups have distinctly lower activation barriers and therefore, proceed preferentially at low temperature. The esterification reaction between the hydrogen bonded carboxylic group and hydroxyl group, together with unimolecular decarboxylation of isolated single carboxylic groups were also possible at moderate temperature. Aryl–aryl coupling is thought to occur via radical pyrolysis and recombination at relatively high temperature.



Similar content being viewed by others
References
Domazetis G, Raoarun M, James BD (2006) Energy Fuels 20:1997–2007
Ozvatan S, Yurum Y (2002) Energy Sources 24:581–589
Li C-Z (2007) Fuel 86:1664–1683
Eskay TP, Britt PF, Buchanan AC (1996) Energy Fuels 10:1257–1261
Ibarra J, Moliner R, Gavilan MP (1991) Fuel 70:408–413
Joseph JT, Forrai TR (1992) Fuel 71:75–80
Manion JA, McMillen DF, Malhotra R (1996) Energy Fuels 10:776–788
Eskay TP, Britt PF, Buchanan AC (1997) Energy Fuels 11:1278–1287
Mae K, Maki T, Okutsu H, Miura K (2000) Fuel 79:417–425
Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I,Martin RL, Fox DJ, Keith T, Al-LahamMA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03, Revision A.1. Gaussian Inc, Pittsburgh PA
Ding L, Fang WH (2010) J Org Chem 75:1630–1636
Peng C, Ayala PY, Schlegel HB, Frisch MJ (1996) J Comput Chem 17:49–56
Peng C, Schlegel HB (1993) Isr J Chem 33:449–454
Gonzalez C, Schlegel HB (1990) J Phys Chem 94:5523–5527
Li J, Brill TB (2003) J Phys Chem A 107:2667–2673
Davidson D, Newman P (1952) J Am Chem Soc 74:1515–1516
Mae K, Maki T, Miura K (2002) J Chem Eng Jpn 35:778–785
Britt PF, Mungall WS, Buchanan AC (1998) Energy Fuels 12:660–661
Ross DS, Loo BH, Tse DS, Hirschon AS (1991) Fuel 70:289–295
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, S., Zhang, Z. & Wang, H. Quantum chemical investigation of the thermal pyrolysis reactions of the carboxylic group in a brown coal model. J Mol Model 18, 359–365 (2012). https://doi.org/10.1007/s00894-011-1077-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-011-1077-5