Abstract
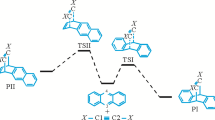
The mechanism of transformation of two radicals (R1p and R1i) obtained by addition of a hydrogen atom to an external and internal carbon atom of dicyclopenta[de,mn]anthracene (P1) was investigated. Two pathways were revealed. The first mechanism is a one-step process, whereas the second mechanism includes two transition states and a cyclobutyl intermediate. The formation of R1p and R1i and the homolytic cleavage of the radicals obtained during the isomerization processes were also examined. In both pathways the addition of a hydrogen atom to the internal carbon significantly lowers the activation energy for hydrogen-mediated isomerization of P1 to acefluoranthene. This finding could be explained by the specific electronic structures of the transition states and intermediates participating in the isomerization processes.

Addition of hydrogen atom to an internal carbon lowers the activation barrier for the Stone-Wales rearrangement








Similar content being viewed by others
References
Otero-Lobato MJ, Kaats-Richters VEM, Havenith RWA, Jenneskens LW, Seinen W (2004) Di-epoxides of the three isomeric dicyclopenta-fused pyrenes: ultimate mutagenic active agents. Mutat Res 564:39–50
Wang JS, He X, Mulder PPJ, Boere BB, Cornelisse J, Lugtenburg J, Busby WF (1999) Comparative tumorigenicity of the cyclopenta-fused polycyclic aromatic hydrocarbons aceanthrylene, dihydroaceanthrylene and acephenanthrylene in preweanling CD-1and BLU: Ha mouse bioassays. Carcinogenesis 20:1137–1141
Howard JB, Longwell JP, Marr JA, Pope CJ, Busby WF Jr, Lafleur AL, Taghizadeh K (1995) Effects of PAH isomerizations on mutagenicity of combustion products. Combust Flame 101:262–270
Scott LT, Necula A (1997) Thermal migration of an ethynyl group from one benzene ring to another by reversible vinylidene C-H insertion. Tetrahedron Lett 38:1877–1880
Sarobe M, Jenneskens LW, Wesseling J, Snoeijer JD, Zwikker JW, Wiersum UE (1997) Thermal interconversions of the C16H10 cyclopenta-fused polycyclic aromatic hydrocarbons fluoranthene, acephenanthrylene, and aceanthrylene revisited. Liebigs Ann/Recueil 6:1207–1213
Sarobe M, Kwint HC, Fleer T, Havenith RWA, Jenneskens LW, Vlietstra EJ, van Lenthe JH, Wesseling J (1999) Flash vacuum thermolysis of acenaphtho[1, 2-a]acenaphthylene, fluoranthene, benzo[k]- and benzo[j]fluoranthene – homolytic scission of carbon-carbon single bonds of internally fused cyclopenta moieties at T ≥ 1100 °C. Eur J Org Chem 5:1191–1200
Necula A, Scott LT (2000) High temperature behavior of alternant and nonalternant polycyclic aromatic hydrocarbons. J Anal Appl Pyrol 54:65–87
Jenneskens LW, Sarobe M, Zwikker JW (1996) Thermal generation and (inter)conversion of (multi) cyclopenta-fused polycyclic aromatic hydrocarbons. Pure Appl Chem 68:219–224
Scott LT, Roelofs NH (1987) Benzene ring contractions at high temperatures. Evidence from the thermal interconversions of aceanthrylene, acephenanthrylene, and fluoranthene. J Am Chem Soc 109:5461–5465
Sarobe M, Jenneskens LW, Wesseling J, Wiersum UE (1997) High temperature gas phase syntheses of C20H12 cyclopenta-fused polycyclic aromatic hydrocarbons: benz[l]-acephenanthrylene and benz[j]acephenanthrylene and their selective rearrangement to benzo[j]fluoranthene. J Chem Soc Perkin Trans 2(4):703–708
Marsh ND, Wornat MJ (2004) Polycyclic aromatic hydrocarbons with five-membered rings: distributions within isomer families in experiments and computed equilibria. J Phys Chem A 108:5399–5407
Scott LT, Roelofs NH (1988) Benzenoid ring contractions in the thermal automerization of acenaphthylene. Tetrahedron Lett 29:6857–6860
Gutman I, Furtula B (2008) Cyclic conjugation in pyracylene. Polyc Arom Comp 28:136–142
Gutman I, Đurđević J (2008) Fluoranthene and its congeners - a graph theoretical study MATCH. Commun Math Comput Chem 60:659–670
Cioslowski J, Schimeczek M, Piskorz P, Moncrieff D (1999) Thermal rearrangement of ethynylarenes to cyclopentafused polycyclic aromatic hydrocarbons: an electronic structure study. J Am Chem Soc 121:3773–3778
Violi A, Sarofim AF, Truong TN (2001) Quantum mechanical study of molecular weight growth process by combination of aromatic molecules. Combust Flame 126:1506–1515
Tsefrikas VM, Scott LT (2006) Geodesic polyarenes by flash vacuum pyrolysis. Chem Rev 106:4868–4884
Marković S, Stanković S, Radenković S, Gutman I (2008) Electronic structure study of thermal intraconversions of some dicyclopenta-fused polycyclic aromatic compounds. J Chem Inf Model 48:1984–1989
Stanković S, Marković S, Radenković S, Gutman I (2009) Formation and isomerization of dicyclopenta[de, mn]anthracene. Electronic structure study. J Mol Model 15:953–958
Stone AJ, Wales DJ (1986) Theoretical studies of icosahedral C60 and some related species. Chem Phys Lett 128:501–503
Murry RL, Strout DL, Odon GK, Scuseria GE (1993) Role of sp3 carbon and 7-membered rings in fullerene annealing and fragmentation. Nature 366:665–667
Balaban AT, Schmalz TG, Zhu H, Klein DJ (1996) Generalizations of the Stone-Wales rearrangement for cage compounds, including fullerenes. J Mol Struct: THEOCHEM 363:291–301
Scott LT (1996) Fragments of fullerenes: novel syntheses, structures and reactions. Pure Appl Chem 68:291–300
Eggen BR, Heggie MI, Jungnickel G, Latham CD, Jones R, Briddon PR (1996) Autocatalysis during fullerene growth. Science 272:87–89
Slanina Z, Zhao X, Uhlík F, Ozawa M, Osawa E (2000) Computational modeling of the elemental catalysis in the Stone-Wales fullerene rearrangements. J Organomet Chem 599:57–61
Alder RW, Harvey JN (2004) Radical-promoted Stone-Wales rearrangements. J Am Chem Soc 126:2490–2494
Slanina Z, Zhao X, Uhlík F, Adamowicz L, Lee S-L (2004) Computations of the catalytic effects in the Stone-Wales fullerene isomerizations: N and CN agents. Int J Quantum Chem 99:634–639
Nimlos MR, Filley J, McKinnon JT (2005) Hydrogen atom mediated Stone-Wales rearrangement of pyracyclene: a model for annealing in fullerene formation. J Phys Chem 109:9896–9903
Marković S, Stanković S, Radenković S, Gutman I (2009) Thermal isomerization in cyclopenta[fg]aceanthrylene. Monats Chem 140:153–156
Richter T, Howard JB (2000) Formation of polycyclic aromatic hydrocarbons and their growth to soot – a review of chemical reaction pathways. Prog Energy Combust Sci 26:565–608
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Becke AD (1993) Density-functional thermochemistry. II. The role of exact exchange. J Chem Phys 98:5648–5652
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA Jr, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick AD, Rabuck KD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (2003) Gaussian 03, Revision E.01-SMP. Gaussian Inc, Pittsburgh, PA
Gonzalez C, Schlegel HB (1989) An improved algorithm for reaction path following. J Chem Phys 90:2154–2161
Foster JP, Weinhold F (1980) Natural hybrid orbitals. J Am Chem Soc 102:7211–7218
Glendening D, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Weinhold F, Copyright 1996–2001 by Board of Regents of the University of Wisconsin System
Zhao Y, Truhlar DG (2004) Hybrid meta density functional theory methods for thermochemistry, thermochemical kinetics, and noncovalent interactions: the MPW1B95 and MPWB1K models and comparative assessments for hydrogen bonding and van der Waals interactions. J Phys Chem A 108:6908–6918
Acknowledgments
This work is supported by the Ministry of science of Serbia, projects No 144015G and 142025.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stanković, S., Marković, S., Gutman, I. et al. Hydrogen-mediated Stone-Wales isomerization of dicyclopenta[de,mn]anthracene. J Mol Model 16, 1519–1527 (2010). https://doi.org/10.1007/s00894-010-0669-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-010-0669-9