Abstract
Predictive pharmacophore models have been developed for a series of arylamino-substituted benzo[b]thiophenes exhibiting free radical scavenging activity. 3D pharmacophore models were generated using a set of 20 training set compounds and subsequently validated by mapping 6 test set compounds using Discovery Studio 2.1 software. Further model validation was performed by randomizing the data using Fischer’s validation technique at the 95% confidence level. The most predictive pharmacophore model developed using the conformers obtained from the BEST method showed a correlation coefficient (r) of 0.942 and consisted of three features: hydrogen bond donor, hydrogen bond acceptor and aromatic ring. Acceptable values of external validation parameters, like \( R_{{\rm{pred}}}^2 \) (0.853) and \( r_{m\left( {test} \right)}^2 \) (0.844), also implied that the external predictivity of the model was significant. The development of further pharmacophore models using conformers obtained from the FAST method yielded a few models with good predictivity, with the best one (r = 0.904) consisting of two features: hydrogen bond donor and hydrogen bond acceptor. Significant values of external validation parameters, \( R_{{\rm{pred}}}^2 \) (0.913) and \( r_{m\left( {test} \right)}^2 \) (0.821), also reflect the high predictive ability of the model. Again, Fischer validation results implied that the models developed were robust enough and their good results were not based on mere chance. These validation approaches indicate the reliability of the predictive abilities of the 3D pharmacophore models developed here, which may thus be further utilized as a 3D query tool in the virtual screening of new chemical entities with potent antioxidant activities.

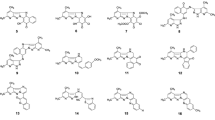
Pharmacophore obtained from hypothesis 1 using the training set conformers developed from the BEST method of conformer generation (Shown are ring aromatic sphere (orange), hydrogen bond donor (magenta) and hydrogen bond acceptor (green) features with vectors in the direction of putative hydrogen bonds)




Similar content being viewed by others
References
Genestra M (2007) Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell Signal 19:1807–1819
Dröge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95
Bayol-Denizot C, Daval J-L, Netter P, Minn A (2000) Xenobiotic-mediated production of superoxide by primary cultures of rat cerebral endothelial cells, astrocytes, and neurons. Biochim Biophys Acta-Mol Cell Res 1497:115–126
Gutteridge JMC, Halliwell B (1994) Antioxidants in nutrition, health and disease. Oxford University Press, Oxford
Wright JS, Johnson ER, DiLabio GA (2001) Predicting the activity of phenolic antioxidants: theoretical method, analysis of substituent effects, and application to major families of antioxidants. J Am Chem Soc 123:1173–1183
Vafiadis AP, Bakalbassis EG (2005) A DFT study on the deprotonation antioxidant mechanistic step of ortho-substituted phenolic cation radicals. Chem Phys 316:195–204
Musialik M, Litwinienko G (2005) Scavenging of dpph• radicals by vitamin E is accelerated by its partial ionization: the role of sequential proton loss electron transfer. Org Lett 7:4951–4954
Helguera AM, Combes RD, Gonzalez MP, Cordeiro MN (2008) Applications of 2D descriptors in drug design: a DRAGON tale. Curr Top Med Chem 8:1628–1655
Gonzalez MP, Teran C, Saiz-Urra L, Teijeira M (2008) Variable selection methods in QSAR: an overview. Curr Top Med Chem 8:1606–1627
Ray S, Sengupta C, Roy K (2007) QSAR modeling of antiradical and antioxidant activities of flavonoids using electrotopological state (E-State) atom parameters. Cent Eur J Chem 5:1094–1113
Ray S, Sengupta C, Roy K (2008) QSAR modeling for lipid peroxidation inhibition of flavonoids using topological and structural parameters. Cent Eur J Chem 6:267–276
Roy K, Mitra I, Saha A (2009) Molecular shape analysis of antioxidant and squalene synthase inhibitory activities of aromatic tetrahydro-1,4-oxazine derivatives. Chem Biol Drug Des 74:507–516
Rastija V, Medic-Saric M (2009) QSAR study of antioxidant activity of wine polyphenols. Eur J Med Chem 44:400–408
Gupta S, Matthew S, Abreu PM, Aires-de-Sousa J (2006) QSAR analysis of phenolic antioxidants using MOLMAP descriptors of local properties. Bioorgan Med Chem 14:1199–1206
Roy K, Mitra I (2009) Advances in quantitative structure–activity relationship models of antioxidants. Expert Opin Drug Discov 4:1157–1175
Wermuth CG, Langer T (2000) Pharmacophore identification. In: Kubinyi H (ed) 3D QSAR in drug design: theory, methods and applications. Kluwer, Dordrecht, pp 117–149
Abreu RMV, Ferreira ICFR, Queiroz MRP (2009) QSAR model for predicting radical scavenging activity of di(hetero)arylamines derivatives of benzo[b]thiophenes. Eur J Med Chem 44:1952–1958
Accelrys Inc. (2010) Discovery Studio 2.1. Accelrys Inc., San Diego
Esteves MA, Narender N, Marcelo-Curto MJ, Gigante B (2001) Synthetic derivatives of abietic acid with radical scavenging ability. J Nat Prod 64:761–766
Thomas JR (1960) The identification of radical products from the oxidation of diphenylamine. J Am Chem Soc 82:5955–5956
Ferreira ICFR, Queiroz MJRP, Vilas-Boas M, Estevinho LM, Begouinb A, Kirsch G (2006) Evaluation of the antioxidant properties of diarylamines in the benzo[b]thiophene series by free radical scavenging activity and reducing power. Bioorg Med Chem Lett 16:1384–1387
Kurogi Y, Guner OF (2001) Pharmacophore modeling and three-dimensional database searching for drug design using catalyst. Curr Med Chem 8:1035–1055
Debnath AK (2002) Pharmacophore mapping of a series of 2,4-diamino-5-deazapteridine inhibitors of mycobacterium avium complex dihydrofolate reductase. J Med Chem 45:41–53
Kahnberg P, Howard MH, Liljefors T, Nielsen M, Nielsen EO, Sterner O, Pettersson I (2004) The use of a pharmacophore model for identification of novel ligands for the benzodiazepine binding site of the GABAA receptor. J Mol Graph Model 23:253–261
Faragalla J, Bremner J, Brown D, Griffith R, Heaton A (2003) Comparative pharmacophore development for inhibitors of human and rat 5-α-reductase. J Mol Graph Model 22:83–92
Ekins S, Bravi G, Wikel JH, Wrighton SA (1999) Three-dimensional-quantitative structure activity relationship analysis of cytochrome P-450 3A4 substrates. J Pharmacol Exp Ther 291:424–433
Leonard JT, Roy K (2006) On selection of training and test sets for the development of predictive QSAR models. QSAR Comb Sci 25:235–251
Everitt B, Landau S, Leese M (2001) Cluster analysis. Arnold, London
Dougherty ER, Barrera J, Brun M, Kim S, Cesar RM, Chen Y, Bittner M, Trent JM (2002) Inference from clustering with application to gene-expression microarrays. J Comput Biol 9:105–126
Accelrys Inc. (2010) Cerius 2, v.4.10. Accelrys Inc, San Diego
Smellie A, Teig SL, Towbin P (1995) Poling: promoting conformational variation. J Comput Chem 16:171–187
Roy K, Paul S (2009) Exploring 2D and 3D QSARs of 2,4-diphenyl-1,3-oxazolines for ovicidal activity against Tetranychus urticae. QSAR Comb Sci 28:406–425
Roy PP, Paul S, Mitra I, Roy K (2009) On two novel parameters for validation of predictive QSAR models. Molecules 14:1660–1701
Mitra I, Saha A, Roy K (2009) Quantitative structure-activity relationship modeling of antioxidant activities of hydroxybenzalacetones using quantum chemical, physicochemical and spatial descriptors. Chem Biol Drug Des 73:526–536
Golbraikh A, Tropsha A (2002) Beware of q2! J Mol Graph Mod 20:269–276
Roy PP, Roy K (2008) On some aspects of variable selection for partial least squares regression models. QSAR Comb Sci 27:302–313
Roy PP, Roy K (2008) Comparative QSAR studies of CYP1A2 inhibitor flavonoids using 2D and 3D descriptors. Chem Biol Drug Des 72:370–382
Patrick GL (2009) An introduction to medicinal chemistry. Oxford University Press, New York
Scott RK, Hoffmann RD Pharmacophoric design and validation for selective D1 agonists. http://accelrys.com/references/case-studies/archive/studies/D1Agonists_full.html. Accessed on February 12, 2010
Hirashima A, Shigeta Y, Eiraku T, Kuwano E (2003) Inhibitors of calling behavior of Plodia interpunctella. J Insect Sci 3:4–12
Acknowledgments
Financial assistance from the All India Council for Technical Education (AICTE), New Delhi in the form of a Research Promotion Scheme is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mitra, I., Saha, A. & Roy, K. Pharmacophore mapping of arylamino-substituted benzo[b]thiophenes as free radical scavengers. J Mol Model 16, 1585–1596 (2010). https://doi.org/10.1007/s00894-010-0661-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-010-0661-4