Abstract
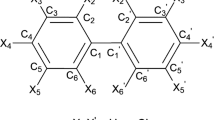
A genetic algorithm was developed and assessed in order to select pairs of proper structural descriptors able to estimate and predict octanol-water partition coefficients of polychlorinated biphenyls (PCBs). The molecular descriptors family was calculated for a sample of 206 PCBs. The problem of searching for the proper descriptors in order to identify structure-activity relationships was translated in genetic terms. The following parameters were imposed in the genetic algorithm (GA) search: sample size − 12, number of variables in multivariate linear regression − 4, imposed adaptation requirements − 3 criteria, maximum number of generations − 50,000, selection strategy − tournament, probability of parent/child mutation − 0.05, number of genes implied in the mutation − 2, optimization parameter - determination coefficient, optimization score - minimum in the sample, and optimization objective - maximum. The highest determination coefficient was obtained in the generation 17,277. Twenty-one evolutions were studied until the optimum solution was obtained. The model identified by the implemented genetic algorithm proved not to be statistically different from the model identified through complete search (ZSteiger = 1.37, p = 0.0861). According to this GA model, the relationship between the structure of PCBs and octanol-water partition coefficients was of geometric and topological nature as previously revealed by the complete search. The genetic algorithm proved its ability to identify two pairs of molecular descriptors able to characterize the relationship between the structure of PCBs and the octanol-water partition coefficient.



Similar content being viewed by others
References
Hansch C, Leo A (1979) Substituent constants for correlation analysis in chemistry and biology. Wiley, New York
Kaminski JJ (1994) Computer-assisted drug design and selection. Adv Drug Deliver Rev 14(2–3):331–337. doi:10.1016/0169-409X(94)90049-3
Barbosa F, Horvath D (2004) Molecular similarity and property similarity. Curr Top Med Chem 4(6):589–600. doi:10.2174/1568026043451186
Baumann K (1999) Uniform-length molecular descriptors for quantitative structure-property relationships (QSPR) and quantitative structure-activity relationships (QSAR): classification studies and similarity searching. Trends Analyt Chem 18(1):36–46. doi:10.1016/S0165-9936(98)00075-2
Bravi G, Gancia E, Mascagni P, Pegna M, Todeschini R, Zaliani A (1997) MS-WHIM, new 3D theoretical descriptors derived from molecular surface properties: A comparative 3D QSAR study in a series of steroids. J Comput Aid Mol Des 11(1):79–92. doi:10.1023/A:1008079512289
Ciubotariu D, Deretey E, Oprea TI, Sulea T, Simon Z, Kurunczi L, Chiriac A (2006) Multiconformational minimal steric difference. Structure-acetylcholinesterase hydrolysis rates relations for acetic acid Esters. QSAR Comb Sci 12(4):367–372. doi:10.1002/qsar.19930120404
Jäntschi L (2005) Molecular descriptors family on structure activity relationships 1. Review of the methodology. Leonardo Electron J Pract Technol 6:76–98 Available via: http://lejpt.academicdirect.org/A06/76_98.htm. Accessed 15 April 2009
Jäntschi L, Bolboacă SD (2007) Results from the use of molecular descriptors family on structure property/activity relationships. Int J Mol Sci 8(3):189–203. doi:10.3390/i8030189
Putz MV, Lacrămă AM (2007) Introducing Spectral Structure Activity Relationship (S-SAR) analysis. Application to ecotoxicology. Int J Mol Sci 8(5):363–469. doi:10.3390/i8050363
Du QS, Huang RB, Wei YT, Pang ZW, Du LQ, Chou KC (2009) Fragment-based quantitative structure-activity relationship (FB-QSAR) for fragment-based drug design. J Comput Chem 30(2):295–304. doi:10.1002/jcc.21056
Du QS, Huang RB, Wei YT, Du LQ, Chou KC (2008) Multiple field three dimensional quantitative structure-activity relationship (MF-3D-QSAR). J Comput Chem 29(2):211–219. doi:10.1002/jcc.20776
Jing JH, Xiao SY, Li ZL (2008) Quantitative structure-activity relationship studies of fatty acids in ranunculus ternatus thunb using three-dimensional holographic vector of atomic interaction field. Fenxi Huaxue/ Chinese J Anal Chem 36(7):971–974
Vedani A, McMasters DR, Dobler M (2000) Multi-conformational ligand representation in 4D-QSAR: Reducing the bias associated with ligand alignment. Quant Struct-Act Relatsh 19(2):149–161. doi:10.1002/1521-3838(200004)19:2<149::AID-QSAR149>3.0.CO;2-9
Vedani A, Dobler M (2002) 5D-QSAR: The key for simulating induced fit? J Med Chem 45(11):2139–2149. doi:10.1021/jm011005p
Eriksson L, Johansson E, Lindgren F, Sjöström M, Wold S (2002) Megavariate analysis of hierarchial QSAR data. J Comput Aided Mol Des 16(10):711–726. doi:10.1023/A:1022450725545
Liang G, Chen G, Niu W, Li Z (2008) Factor analysis scales of generalized amino acid information as applied in predicting interactions between the human amphiphysin-1 SH3 domains and their peptide ligands. Chem Biol Drug Des 71(4):345–351. doi:10.1111/j.1747-0285.2008.00641.x
Tsygankova IG (2008) Variable selection in QSAR models for drug design. Curr Comput Aided Drug Des 4(2):132–142. doi:10.2174/157340908784533238
Khan MTH, Sylte I (2007) Predictive QSAR modeling for the successful predictions of the ADMET properties of candidate drug molecules. Curr Drug Discov Technol 4(3):141–149
Vighi M, Migliorati S, Monti GS (2009) Toxicity on the luminescent bacterium Vibrio fischeri (Beijerinck). I: QSAR equation for narcotics and polar narcotics. Ecotoxicol Environ Saf 72(1):154–161. doi:10.1016/j.ecoenv.2008.05.008
Lin W-Q, Jiang J-H, Wu H-L, Shen G-L, Yu R-Q (2006) Recent advances in chemometric methodologies for QSAR studies. Curr Comput Aided Drug Des 2(3):255–266. doi:10.2174/157340906778226418
Riahi S, Pourbasheer E, Dinarvand R, Ganjali MR, Norouzi P (2008) QSAR Study of 2-(1-Propylpiperidin-4-yl)-1H-Benzimidazole-4-Carboxamide as PARP inhibitors for treatment of cancer. Chem Biol Drug Des 72(6):575–584. doi:10.1111/j.1747-0285.2008.00739.x
Duchowicz PR, Castro EA (2008) Partial order theory applied to QSPR-QSAR studies. Comb Chem High Throughput Screen 11(10):783–793. doi:10.2174/138620708786734316
Xiao Y-D, Harris R, Bayram E, Santago P II, Schmitt JD (2006) Supervised self-organizing maps in drug discovery. 2. Improvements in descriptor selection and model validation. J Chem Inf Model 46(1):137–144. doi:10.1021/ci0500841
Fox T, Kriegl JM (2006) Machine learning techniques for in silico modeling of drug metabolism. Curr Top Med Chem 6(15):1579–1591. doi:10.2174/156802606778108915
Du H, Wang J, Hu Z, Yao X, Zhang X (2008) Prediction of fungicidal activities of rice blast disease based on least-squares support vector machines and project pursuit regression. J Agric Food Chem 56(22):10785–10792. doi:10.1021/jf8022194
George CJ, Bennett GF, Simoneaux D, George WJ (1988) Polychlorinated biphenyls a toxicological review. J Hazard Mater 18(2):113–144. doi:10.1016/0304-3894(88)85018-0
Hansen BG, Paya-Perez AB, Rahman M, Larsen BR (1999) QSARs for K(ow) and K(oc) of PCB congeners: A critical examination of data, assumptions and statistical approaches. Chemosphere 39(13):2209–2228. doi:10.1016/S0045-6535(99)00145-9
Giri S, Roy DR, Van Damme S, Bultinck P, Subramanian V, Chattaraj PK (2008) An atom counting QSPR protocol. QSAR Comb Sci 27(2):208–230. doi:10.1002/qsar.200730109
Ivanciuc T, Ivanciuc O, Klein DJ (2006) Modeling the bioconcentration factors and bioaccumulation factors of polychlorinated biphenyls with posetic quatitative super-structure/activity relationships (QSSAR). Mol Divers 10:133–145. doi:10.1007/s11030-005-9003-3
Jiang GX, Niu JF, Zhang SP, Zhang ZY, Xie B (2008) Prediction of biodegradation rate constants of hydroxylated polychlorinated biphenyls by fungal laccases from Trametes versicolor and Pleurotus ostreatus. Bull Environ Contam Toxicol 81(1):1–6. doi:10.1007/s00128-008-9433-6
Zeng X, Wang Z, Ge Z, Liu H (2007) Quantitative structure-property relationships for predicting subcooled liquid vapor pressure (PL) of 209 polychlorinated diphenyl ethers (PCDEs) by DFT and the position of Cl substitution (PCS) methods. Atmos Environ 41(17):3590–3603. doi:10.1016/j.atmosenv.2006.12.039
Jäntschi L, Bolboacă SD, Diudea MV (2007) Chromatographic retention times of polychlorinated biphenyls: From structural information to property characterization. Int J Mol Sci 8(11):1125–1157. doi:0.3390/i8111125
Wei B, Xie S, Yu M, Wu L (2007) QSPR-based prediction of gas/particle partitioning of polychlorinated biphenyls in the atmosphere. Chemosphere 66(10):1807–1820. doi:10.1016/j.chemosphere.2006.09.029
Borja J, Taleon DM, Auresenia J, Gallardo S (2005) Polychlorinated biphenyls and their biodegradation. Process Biochem 40(6):1999–2013. doi:10.1016/j.procbio.2004.08.006
Hertz-Picciotto I, Charles MJ, James RA, Keller JA, Willman E, Teplin S (2005) In utero polychlorinated biphenyl exposures in relation to fetal and early childhood growth. Epidemiology 16(5):648–656. doi:10.1097/01.ede.0000173043.85834.f3
Bodin N, Le Loc'h F, Caisey X, Le Guellec A-M, Abarnou A, Loizeau V, Latrouite D (2008) Congener-specific accumulation and trophic transfer of polychlorinated biphenyls in spider crab food webs revealed by stable isotope analysis. Environ Pollut 151(1):252–261. doi:10.1016/j.envpol.2007.01.051
Ruiz P, Faroon O, Moudgal CJ, Hansen H, De Rosa CT, Mumtaz M (2008) Prediction of the health effects of polychlorinated biphenyls (PCBs) and their metabolites using quantitative structure-activity relationship (QSAR). Toxicol Lett 181(1):53–65. doi:10.1016/j.toxlet.2008.06.870
Jäntschi L, Bolboacă SD (2006) Molecular Descriptors Family on Structure Activity Relationships 6. Octanol-Water Partition Coefficient of Polychlorinated Biphenyls. Leonardo Electron J Pract Technol 8:71–86 Available via: http://lejpt.utcluj.ro/A08/71_86.htm. Accessed 15 April 2009
Jäntschi L, Bolboacă SD (2007) Integrated Structural Investigations on Biological Active Compounds (Research Report; in Romanian). Available via: http://lori.academicdirect.org/research/grants/Raport_Cercetare_ET036_2007.pdf. Accessed 15 April 2009
Connor MS (1985) Comment on „fish/sediment concentration ratios for organic compounds”. Environ Sci Technol 19(2):198–199. doi:10.1021/es00132a015
Eisler R, Belisle AA (1996) Planar PCB Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review. Contaminant Hazard Reviews 1–96. Available via: http://www.pwrc.usgs.gov/infobase/eisler/chr_31_planar_pcbs.pdf. Accessed 18 April 2009
Hoffmann R (1963) An extended Hückel theory. I. Hydrocarbons. J Chem Phys 39(6):1397–1412. doi:10.1063/1.1734456
Dewar MJS, Zoebisch EG, Healy EF, Stewart JJP (1985) Development and use of quantum mechanical molecular models. 76. AM1: a new general purpose quantum mechanical molecular model. J Am Chem Soc 107(13):3902–3909. doi:10.1021/ja00299a024
Fisher RA (1918) The correlation between relatives on the supposition of Mendelian inheritance. Trans R Soc Edin 52:399–433
Weismann A (1893) The germ-plasm: a theory of heredity. C. Scribner's Sons, New York
de Veies H (1902) The origin of species by mutation. Science 15(384):721–729. doi:10.1126/science.15.384.721
Auerbach C, Robson JM, Carr JG (1947) The chemical production of mutations. Science 105(2723):243–247. doi:10.1126/science.105.2723.243
Cairns J, Overbaugh J, Miller S (1988) The origin of mutants. Nature 335(6186):142–145. doi:10.1038/335142a0
Darwin CR (1859) On the origin of species by means of natural selection. J Murray, London
Jarque CM, Bera AK (1980) Efficient tests for normality, homoscedasticity and serial independence of regression residuals. Econ Lett 6(3):255–259. doi:10.1016/0165-1765(80)90024-5
Kleinbaum DG, Kupper LL, Muller KE, Nizam A (2008) Applied regression analysis and multivariable methods, 4th edn. Duxbury (Thomson Higher Educatio), Canada, pp 141–146
Steiger JH (1980) Tests for comparing elements of a correlation matrix. Psychol Bull 87:245–251
Bolboacă SD, Jäntschi L (2008) Modelling the property of compounds from structure: statistical methods for models validation. Environ Chem Lett 6:175–181. doi:10.1007/s10311-007-0119-9
Daren Z (2001) QSPR studies of PCBs by the combination of genetic algorithms and PLS analysis. Comput Chem 25(2):197–204. doi:10.1016/S0097-8485(00)00081-4
Todeschini R, Consonni V, Mauri A, Pavan M (2004) Detecting "bad" regression models: Multicriteria fitness functions in regression analysis. Anal Chim Acta 515(1):199–208. doi:10.1016/j.aca.2003.12.010
Pavan M, Mauri A, Todeschini R (2004) Total ranking models by the genetic algorithm variable subset selection (GA-VSS) approach for environmental priority settings. Anal Bioanal Chem 380:430–444. doi:10.1007/s00216-004-2762-3
Acknowledgments
UEFISCSU Romania partially supported this research through project (ID-202/01.10.2007 & ID-206/01.10.2007).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Materials
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jäntschi, L., Bolboacă, S.D. & Sestraş, R.E. Meta-heuristics on quantitative structure-activity relationships: study on polychlorinated biphenyls. J Mol Model 16, 377–386 (2010). https://doi.org/10.1007/s00894-009-0540-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-009-0540-z