Abstract
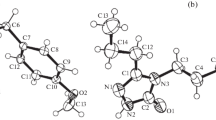
The title compound, methyl 2-methoxy-7-(4-methylbenzoyl)-4-oxo-6-p-tolyl-4H-furo[3,2-c]pyran-3-carboxylate (C25H20O7), was prepared and characterized by IR and single-crystal X-ray diffraction (XRD). The compound crystallizes in the triclinic space group P −1 with a = 8.9554(9) Å, b = 10.0018(10) Å, c = 12.7454(13) Å, α = 67.678(7)°, β = 89.359(8)° and γ = 88.961(8)°. In addition to the molecular geometry from X-ray experiment, the molecular geometry and vibrational frequencies of the title compound in the ground state have been calculated using semiempirical AM1 and PM3 methods, as well as Hartree-Fock (HF) and density functional (B3LYP) levels of theory with 6–31G(d) basis set. To determine conformational flexibility, molecular energy profile of the title compound was obtained by semi-empirical (AM1) calculations with respect to two selected degrees of torsional freedom, which were varied from −180° to +180° in steps of 10°. Besides, frontier molecular orbitals (FMO) analysis and thermodynamic properties of the title compound were performed by the B3LYP/6–31G(d) method.







Similar content being viewed by others
References
Mo S, Wang S, Zhou G, Yang Y, Li Y, Chen X, Shi J (2004) J Nat Prod 67:823–828
Kim JP, Yun B-S, Shim YK, Yoo ID (1999) Tetrahedron Lett 40:6643–6644
Dinçer M, Yıldırım İ, Koca İ, Özdemir N (2004) Acta Cryst E60:0207–0209
Florey HW, Chain E, Headley NG, Jennings M, Sanders AG, Abraham ED, Florey ME (1949) Antibiotics, 2 vols. Oxford Univ Press, London
Woordward RB, Singh G (1950) J Am Chem Soc 72(3):1428
Suarez M, Salfran E, Verdecia Y, Ochoa E, Alba L, Martin N, Martinez R, Quinteiro M, Seoane C, Novoa H, Blaton N, Peeters OM, De Ranter C (2002) Tetrahedron 58:953–960
Kemnitzer W, Drewe J, Jiang S, Zhang H, Wang Y, Zhao J, Jia S, Herich J, Labreque D, Storer R, Meerovitch K, Bouffard D, Rej R, Denis R, Blais C, Lamothe S, Attardo G, Gourdeau H, Tseng B, Kasibhatla S, Cai SX (2004) J Med Chem 47:6299–6310
Zhang H-Z, Kasibhatla S, Kuemmerle J, Kemnitzer W, Ollis-Mason K, Qiu L, Crogan-Grundy C, Tseng B, Drewe J, Cai SX (2005) J Med Chem 48:5215–5223
Andreani LL, Lapi E (1960) Boll Chim Farm 99:583–587
Bonsignore L, Loy G, Secci D, Calignano A (1993) Eur J Med Chem 28:517–519
Kojima K, Ohno T, Inoue M, Mizukami H, Nagatsu A (2008) Chem Pharm Bull 56(2):173–175
Armetso D, Horspool WM, Martin N, Ramos A, Seaone C (1989) J Org Chem 54:3069–3072
Hatakeyama S, Ochi N, Numata H, Takano S (1988) J Chem Soc Chem Commun 17:1202–1204
Gonzalez R, Martin N, Seoane C, Marco JL, Albert A, Cano FH (1992) Tetrahedron Lett 33:3809–3812
Madhushaw RJ, Li CL, Shen KH, Hu CC, Liu RS (2001) J Am Chem Soc 123(30):7427–7428
Shen KH, Lush SF, Chen TL, Liu RS (2001) J Org Chem 66:8106–8111
Snider BB, Roush DM, Killinger TA (1979) J Am Chem Soc 101:6023–6027
Fuhrer C, Messer R, Häner R (2004) Tetrahedron Lett 45:4297–4300
Takano S, Satoh S, Ogasawara K, Aoe K (1990) Heterocycles 30:583–605
Ziegler E, Eder M, Belegratis C, Prewedourakis E (1967) Monatsh Chem 98:2249–2251
Yıldırım İ, Koca İ (2005) Kuwait J Sci Eng 32(1):49–60
Yıldırım İ, Koca İ, Dinçer M (2008) J Chem Soc Pak 30(1):134–141
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Becke AD (1993) J Chem Phys 98:5648–5652
Ditchfield R, Hehre WJ, Pople JA (1971) J Chem Phys 54:724–728
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03. Revision E.01. Gaussian Inc., Wallingford CT
Foresman JB, Frisch A (1996) Exploring chemistry with electronic structure methods, 2nd edn. Gaussian Inc, Pittsburgh
Dennington R II, Keith T, Millam J (2007) GaussView, Version 4.1.2. Semichem Inc, Shawnee Mission, KS
Farrugia LJ (1997) J Appl Crystallogr 30:565
Bucourt R (1974) In: Eliel EL, Allinger N (eds) Topics in Stereochemistry, vol 8. John Wiley, New York, pp 159–224
Bruno G, Nicoló F, Rotondo A, Foti F, Risitano F, Grassi G, Bilardo C (2001) Acta Cryst C57:493–494
Bernstein J, Davis RE, Shimoni L, Chang N-L (1995) Angew Chem Int Ed Engl 34:1555–1573
Allen FH (2002) Acta Cryst B58:380–388
Bruno IJ, Cole JC, Edgington PR, Kessler M, Macrae CF, McCabe P, Pearson J, Taylor R (2002) Acta Cryst B58:389–397
Acknowledgments
This study was supported financially by the Research Centre of Ondokuz Mayıs University (Project No: F-425).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Özdemir, N., Dinçer, M., Koca, İ. et al. Methyl 2-methoxy-7-(4-methylbenzoyl)-4-oxo-6-p-tolyl-4H-furo[3,2-c]pyran-3-carboxylate:A combined experimental and theoretical investigation. J Mol Model 15, 1193–1201 (2009). https://doi.org/10.1007/s00894-009-0479-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-009-0479-0