Abstract
Cytochrome P-450 is a group of enzymes involved in the biotransformation of many substances, including drugs. These enzymes possess a heme group (1) that when it is properly modified induces several important physicochemical changes that affect their enzymatic activity. In this work, the five structurally modified heme derivatives 2–6 and the native heme 1 were docked on CYP2B4, (an isoform of P450), in order to determine whether such modifications alter their binding form and binding affinity for CYP2B4 apoprotein. In addition, docking calculations were used to evaluate the affinity of CYP2B4 apoprotein-heme complexes for aniline (A) and N-methyl-aniline (NMA). Results showing the CYP2B4 heme 4- and heme 6-apoprotein complexes to be most energetically stable indicate that either hindrance effects or electronic properties are the most important factors with respect to the binding of heme derivatives at the heme-binding site. Furthermore, although all heme-apoprotein complexes demonstrated high affinity for both A and NMA, the CYP2B4 apoprotein-5 complex had higher affinity for A, and the heme 6 complex had higher affinity for NMA. Finally, surface electronic properties (SEP) were calculated in order to explain why certain arginine residues of CYP2B4 apoprotein interact with polarizable functionalities, such as ester groups or sp 2 carbons, present in some heme derivates. The main physicochemical parameter involved in the recognition process of the heme derivatives, the CYP2B4 apoprotein and A or NMA, are reported.

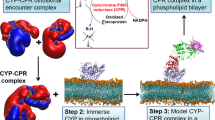
Scheme of steps to be followed for obtaining five new CYP2B4 apoprotein-heme complexes by docking






Similar content being viewed by others
References
Fleming BD, Johnson DL, Bond AM, Martin LL (2006) Expert Opin Drug Metab Toxicol 2:581–589
Lamb DC, Kim Y, Yermalitskaya LV, Yermalitsky VN, Lepesheva GI, Kelly SL, Waterman MR, Podust LM (2006) Structure 14:51–61
Hlavica P, Lehnerer M, Eulitz M (1996) Biochem J 318:857–862
Gutierrez A, Grunau M, Paine AW, Munro CR, Wolf GC, Roberts NS (2003) Biochem Soc Trans 31:497–501
Zhao X, Yeung N, Wang Z, Guo Z, Lu Y (2005) Biochem J 44:1210–1214
Uchida T, Ishimori K, Morishima I (1997) J Biol Chem 272:30108–30114
Aschi M, Zazza C, Spezia R, Bossa C, Di Nola A, Paci M, Amadei A (2004) J Comput Chem 25:974–984
Galstyan AS, Zaric SD, Knapp EW (2005) J Biol Inorg Chem 10:343–354
Mie Y, Yamada C, Hareau GP, Neya S, Uno T, Funasaki N, Nishiyama K, Taniguchi I (2004) Biochem J 43:13149–13155
Torres E, Baeza A, Vazquez-Duhalt R (2002) J Mol Catal B: Enzymatic 19–20:437–441
Sono M, Asakura T (1996) J Biol Chem 251:2664–2670
Rosales-Hernández M, Kispert L, Torres-Ramírez E, Ramírez-Rosales D, Zamorano- Ulloa R, Trujillo-Ferrara J (2007) Biotechnol Lett 29:919–924
Cheng D, Reed JR, Harris D, Backes WL (2007) Arch Biochem Biophys 462:28–37
Zhao Y, Halpert JR (2007) Biochem Biophys Acta 1770:402–412
Rosales-Hernández MC, Correa-Basurto J, Flores-Sandoval C, Marín-Cruz J, Torres E, Trujillo-Ferrara J (2007) J Mol Struct THEOCHEM 804:81–88
Lee K-B, Jun E, La Mar GN, Rezzano IN, Pandey RK, Smith KM, Walker FA, Buttlaire DH (1991) J Am Chem Soc 113:3576–3583
Tsukahara K, Okazawa T, Takahashi H, Yamamoto Y (1986) Inorg Chem 25:4756–4760
Singh UP, Obayashi E, Takahshi S, Lizuka T, Shoun H, Shiro Y (1998) Biochem Biophys Acta 1384:103–111
Hudecek J, Hodek P, Anzenbacherova E, Anzenbacher P (2007) BBA-General Subjects 1770:413–419
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Peterson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (1998) Gaussian 98, Revision A.9. Gaussian Inc, Pittsburgh PA
Frankcombe KE, Cavell KJ, Yates BF, Knott RB (1995) J Phys Chem 99:14316–14322
Zhao Y, White MA, Muralidhara BK, Sun L, Halpert JR, Stout CD (2005) J Biol Chem 281:5973–5981
Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ (1998) J Comp Chem 19:1639–1662
Goodford PJ (1985) J Med Chem 28:849–857
Humphrey W, Dalke A, Schulten K (1996) J Mol Graph 14:33–38
Cios KJ, Mamitsuka H, Nagashima T, Tadeusiewicz R (2005) Artif Intell Med 35:1–8
Correa-Basurto J, Flores-Sandoval C, Marín-Cruz J, Rojo-Domínguez A, Espinoza- Fonseca LM, Trujillo-Ferrara JG (2007) Eur J Med Chem 42:10–19
Knops-Gerrits PP, Jacobs PA, Fukuoka A, Ichikawa M, Faglioni F, Goddard WA (2001) J Mol Catal A: Chemical 166:3–13
Sivozhelezov V, Pechkova E, Nicolini C (2006) J Theoretical Biol 241:73–80
Stjernschantz E, Marelius J, Medina C, Jacobsson M, Vermeulen NPE, Oostenbrink C (2006) J Chem Inf Model 46:1972–1983
De Groot MJ, Kirton SB, Sutcliffe MJ (2004) Curr Top Med Chem 4:1803–1824
Vahedi-Faridi A, Brault PA, Shah P, Kim YW, Dunham WR, Funk MO (2004) J Am Chem Soc 126:2006–2015
Pearce RE, Leeder JS, Kearns GL (2006) Drug Metab Dispos 34:1035–1040
Arnold F, Weigend F (2007) J Chem Physics 126:174101–174115
Ferro N, Tacoronte JE, Reinard T, Bultinck P, Montero LA (2006) J Mol Struct THEOCHEM 758:263–274
Zhu Y, Silverman RB (2007) J Org Chem 72:233–239
Zhang Y, Yao P, Cai X, Xu H, Zhang X, Jiang J (2007) J Mol Graph Model 26:319–326
Lill MA, Dobler M, Vedani A (2006) Chem Med Chem 1:73–81
Pichierri F (2004) Biophys Chem 109:295–304
Trogdon G, Murray JS, Concha MC, Politzer P (2007) J Mol Model 13:313–318
De Visser SP (2006) J Am Chem Soc 128:15809–15818
Hlavica P (1972) Biochem Biophys Acta 273:318–327
Flores-Sandoval CA, Zaragoza IP, Maranon-Ruiz VF, Correa-Basurto J, Trujillo-Ferrara J (2005) J Mol Struct THEOCHEM 713:127–134
Wojciechowski PM, Zierkiewicz W, Michalska D, Hobza P (2003) J Chem Physics 118:10900–10911
Muralidhara BK, Negi S, Chin CC, Braun W, Halpert JR (2006) J Biol Chem 281:8051–8061
Acknowledgements
The authors thank CONACYT (62488) and SIP-COFAA/IPN (20070140) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mendieta-Wejebe, J.E., Rosales-Hernández, M.C., Rios, H. et al. Comparing the electronic properties and docking calculations of heme derivatives on CYP2B4. J Mol Model 14, 537–545 (2008). https://doi.org/10.1007/s00894-008-0294-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-008-0294-z