Abstract
The hydroxycinnamyl alcohols: p-coumarol, coniferol and sinapol are considered the basic units and precursors of lignins models. In this work, the specific reactivity of these molecules was studied. We investigate their intrinsic chemical reactivity in terms of the Fukui function, applying the principle of hard and soft acids and bases (HSAB) in the framework of the density functional theory (DFT). Comparisons of their nucleophilic, electrophilic and free radical reactivity show their most probably sites to form linkages among them. It is found that the most reactive sites, for reactions involving free radicals, are the carbons at the β-position in the p-coumarol and sinapol molecules, whilst the regions around the carbon-oxygen bond of the phenoxyl group are the most reactive in coniferol.

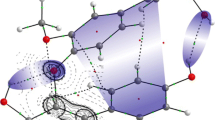
Isocontour plots for the free radical form of the Fukui function f 0 (r), showing the reactive sites toward electron-rich/poor reactants





Similar content being viewed by others
References
Sakakibara A, Sano Y (2001) Lignin. In: Hon DNS, Shirasi N (eds) Wood and cellulosic chemistry, chapter 4. CRC, New York, pp 109–174
Freudenberg K (1965) Science 148:595–600
Monties B (1989) Plant phenolics. In: Dey PM, Harborne JB (eds) Methods in plant biochemistry. Academic, New York, pp 113–157
Higuchi T (2006) Wood Sci Technol 40:16–25
Thielemans W, Wool RP (2005) Biomacromolecules 6:1895–1905
John R, Yinsheng Z (1998) Tetrahedron 58:1349–1354
Liu R, Zhou X (1993) J Phys Chem 97:9613–9617
Glasser WG, Sarkanen S (1989) Lignin, properties and materials. American Chemical Society, Washington, DC, pp 64–113
Jurasek L (1995) J Pulp Paper Sci 21:274–279
Houtman CJ, Atalla RH (1995) Plant Physiol 107:977–984
Simon JP, Eriksson LA (1995) Holzforschung 49:429–438
Elder TJ, McKee ML, Worley SD (1988) Holzforschung 42:233–240
Besombes S, Robert D, Utille JP, Taravel F, Mazeau K (2003) J Agric Food Chem 51:34–42
Durbeej B, Eriksson LA (2003) Holzforschung 57:466–478
Pearson RG (1963) J Am Chem Soc 85:3533–3539
Beck ME (2005) J Chem Inf Model 45:273–282
Ayers PW, Parr RG (2000) J Am Chem Soc 122:2010–2018
Parr RG, Yang W (1989) Density functional theory of atoms and molecules. Oxford University Press, New York, pp 70–84
Parr RG, Pearson RG (1983) J Am Chem Soc 105:7512–7516
Parr RG, Donnelly RA, Levy M, Palke WE (1978) J Chem Phys 68:3801–3807
Yang W, Parr RG (1985) Proc Natl Acad Sci USA 82:6723–6726
Parr RG, Yang W (1984) J Am Chem Soc 106:4049–4050
Chattaraj PK, Lee H, Parr RG (1991) J Am Chem Soc 113:1855–1856
Gázquez JL (1993) Chemical hardness. In: Sen KD (ed) Structure and bonding 80:27–42
von Barth U, Gelatt CD (1980) Phys Rev B 21:2222–2228 and references therein
Herrera H, Nagarajan A, Morales MA, Mendez F, Jiménez HA, Zepeda LG, Tamariz J (2001) J Org Chem 66:1252–1263
López P, Méndez F (2004) Org Lett 6:1781–1783
Nemukhin AV, Grigorenko BL, Granovsky A (2004) Mosc Univ Chem Bull 45:75–78
Becke AD (1993) J Chem Phys 98:5648–5652
Portmann S, Flükiger HP, Weber J (2000) Swiss center for scientific computing, Manno Switzerland
Sakakibara A (1980) Wood Sci Technol 14:89–100
Acknowledgements
This work was supported by the Graduate College at the Faculty of Wood Technology, and Engineering (FITECMA) at the Universidad Michoacana de San Nicolas de Hidalgo (UMSNH) and The Michoacan State Science and Technology Council (COECYT-Michoacan).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martinez, C., Rivera, J.L., Herrera, R. et al. Evaluation of the chemical reactivity in lignin precursors using the Fukui function. J Mol Model 14, 77–81 (2008). https://doi.org/10.1007/s00894-007-0253-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-007-0253-0