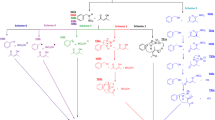
Abstract
Full geometric optimization of endo-tricyclo[3.2.1.02,4]oct-6-ene (endo-TCO) by ab initio and DFT methods allowed us to investigate the structure of the molecule. The double bond is endo-pyramidalized and its two faces are no longer found to be equivalent. The exo face of the double bond has regions with far more electron density (qi,HOMO) and more negative electrostatic potential. The endo-TCO-Br2 system was investigated at the B3LYP/6-311+G** level and the endo-TCO···Br2(exo) molecular complex was found to be relatively more stable than the endo-TCO···Br2(endo) complex. The cationic intermediates of the reaction were studied by ab initio and DFT methods. The bridged exo-bromonium cation(I) is relatively more stable than the endo-bromonium cation(II). An absolute exo-facial selectivity should be observed in the addition reaction of Br2 to endo-TCO, which is caused by steric and electronic factors. The nonclassical rearranged cation IV was found to be the most stable ion among the cationic intermediates and the ionic addition occurs via the formation of this cation. The mechanism of the addition reaction is also discussed.





Similar content being viewed by others
References
Belluci G, Chappe C, Bianchini R, Lenoir D, Herges R (1995) J Am Chem Soc 117:12001–12002
Herges R (1995) Angew Chem Int Ed Engl 34:51–53
Ruiz E, Dennis R, Salahub R, Vela A (1996) J Phys Chem 100:12265–12276
Brown RS (1997) Acc Chem Res 30:131–137
Bianchini R, Chappe C, Lenoir D, Lammeu R, Herges R, Grunenber J (1997) Angew Chem Int Ed Engl 36:1284–1287
Bianchini R, Chiappe C, Moro LG, Lenoir D, Lemmen P, Goldberg N (1999) Chem Eur J 5:1570–1580
Chiappe C, Rubertis AD, Lemmen P, Lenoir D (2000) J Org Chem 65:1273–1279
Legon AC, Thumwood JMA (2001) Phys Chem Chem Phys 3:1397–1403
Chiappe C, Rubertis AD, Lemmen D, Jaber A, Lenoir D, Watteubochi C, Ponelli CS (2002) J Org Chem 67:7066–7074
Smith WB (1998) J Org Chem 63:2661–2664
Chiappe C, Rubertis DA, Detert H, Lenoir D, Wannere C, Schleyer RP (2002) Chem Eur J 8:967–978
Rathere R, Lindeman SV, Zhu CJ, Mori T, Schleyer RP, Kochi JK (2002) J Org Chem 67:5106–5116
Lenoir D, Chiappe C (2003) Chem Eur J 9:1037–1044
Chiappe C, Detert H, Lenoir D, Pamelli CS, Ruasse MF (2003) J Am Chem Soc 125:2864–2865
Abbasoglu R (2004) J Mol Struct (Theochem) 686:1–5 and references therein
Coxon JM, Steel PJ, Burritt A, Whittington BI (1995) Tetrahedron 51:8057–8072
Dastan A, Demir U, Balci M (1999) J Org Chem 59:6534–6538
Burritt A, Coxon JM, Steel PJ (1996) J Org Chem 61:4328–4335
Satake K, Hikasa K, Itoh H, Okamoto H, Kimura M, Morosawa S (1996) Bull Chem Soc Jap 69:453–457
Dastan A (2001) Tetrahedron 57:8725–8732
Horasan N, Kara Y, Azizoglu A, Balci M (2003) Tetrahedron 59:3691–3699
Hehre WJ, Ditchfield R, Pople JA (1972) J Chem Phys 56:2257–2261
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) J Chem Phys 72:650–654
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Frisch MJ, Pople JA, Binkley JS (1984) J Chem Phys 80:3265–3269
Krishnan R, Frisch MJ, Pople JA, (1980) J Chem Phys 72:4244–4249
Borden WT (1989) Chem Rev 89:1095–1109
Ermer O, Bell P, Mason SA (1989) Angew Chem Int Ed Engl 28:1239–1241
Watson WH (1983) Stereochemistry and reactivity of systems containing ( electrons. Verlag Chemie International, Deerfield beach, Florida
Houk KN, Rondon NG, Brown FK, Jorgensen WL, Madura JD, Spellmeyer DC (1983) J Am Chem Soc 105:5980–5988
Gandalfi R, Tonaletti G, Rostelli A, Bagatti M (1993) J Org Chem 58:6038–6048
Royer J (1978) Tetrahedron Lett 1343–1346
Paguette LA, Belliamy F, Wells GJ, Bohm MC, Gleiter R (1981) J Am Chem Soc 103:7122–7133
Broughton HB, Green SM, Rzepa HS (1992) J Am Chem Soc, Chem Commun 998–1001
Fleming I (1976) Frontier orbitals and organic chemical reactions. Wiley, New York, pp 5–33
Smirnov VV, Tihomirov VA, Cudinov GE (1993) Zh Struct Chem 34:14–18
Teberekidis VI, Sigalas MP (2002) Tetrahedron 58:6171–6178
Teberekidis VI, Sigalas MP (2003) Tetrahedron 59:4749–4751
Perera SA, Bartlett RJ (1996) J Am Chem Soc 1118:7849–7850
Werstiuk NH, Muchall HM (1999) J Mol Struct (Theochem) 463:225–229
Werstiuk NH, Muchall HM (2000) J Phys Chem 104A: 2054–2060
Werstiuk NH, Wang YG (2001) J Phys Chem 105A:11515–1152
Cremer D, Childs RL, Kraka E (1995) In the chemistry of the cyclopropyl group. In: Rappoport Z (ed) Wiley, New York, 2:339–410
de la Mare PBD, Bolton R (1982) Electrophilic additions to unsaturated systems, 2nd edn. Elsevier, New York, pp 135–197
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abbasoglu, R., Yilmaz, S.S. Ab initio and DFT study on the electrophilic addition of bromine to endo-tricyclo[3.2.1.02,4]oct-6-ene. J Mol Model 12, 290–296 (2006). https://doi.org/10.1007/s00894-005-0031-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-005-0031-9