Abstract
This work describes a theoretical approach to the substitution reaction mechanism involving the conversion of cholesterol to cholesteryl chloride. Two chlorosulfite ester molecules were formed as intermediates. An iso-steroid was found as the transition state. The final product was cholesteryl chloride and the side products were HCl and SO2. Calculations were carried out at high level Hartree–Fock theory, using the 6–31G* basis set. From the electronic structure of the reactants, the most important physicochemical properties involved in the reaction pathway were used. Thus, to determine the participation of each molecule and to explain the mechanism of reaction; the total energy, HOMO and LUMO, atomic orbital contribution to frontier orbitals formation, electrostatic potentials, atomic charges, hardness and dipole moment were used. Characterization of intermediates and transition state was supported by their respective energy minima, fundamental frequencies and equilibrium geometry.
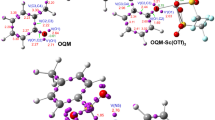
Figure Synopsis of the reaction pathway. The reaction starts when the lone pair of the Ch oxygen interacts with the sulfur atom, releasing a chloride ion. As a result, the first intermediate is formed. Next, in the first intermediate the nucleophilic chloride ion bonds the electrophilic hydrogen atom, releasing HCl and yielding the second intermediate. In the second intermediate, the electrophilic H-atom from HCl bonds with the lone pair of the Cl atom adjacent to the sulfur atom, restoring HCl. Concurrently, SO2 is liberated and causes the formation of the C3-C5 partial bond and breaking of the C5-C6 π-bond leading to the transition state. In the transition state, the electrophilic H from HCl bonds with the Cl lone pair at C6-Cl, forming HCl again and leaving the C6 atom electron-deficient, which restores the C5-C6 π-bond and breaks the C3-C5 partial bond. Finally, the electrophilic C3 atom and the nucleophilic Cl atom form a bond, yielding cholesteryl chloride. HCl and SO2 are also formed as side products. The arrows show the rearrangement of electrons.







Similar content being viewed by others
References
Sharma K, Saleem K, Salim A (2002) Indian J Chem 41B:2676–2678
Sharma K, Saleem K, Salim A (2003) Indian J Chem 42B:2866–2868
Marker RE, Kamm O, Oakwood TS, Laucius JF (1936) J Am Chem Soc 58:1948–1950
Diels O, Abderhalden E (1904) Ber 37:3092–3103
Spartan Version 1.0.0 (2003) Wavefunction Inc., 18401 Von Karman Avenue, Suite 370, Irvine, CA 92612, USA
Cabrera-Vivas BM, Ramírez JC, Martínez-Aguilera LMR, Kubli-Garfias C (2002) J Mol Struct, (Theochem) 584:5–14
Shoppee CW, Summers GHR (1952) J Chem Soc:3361–3374
Fukui K (1982) Science 218:747–754
Pearson RG (1987) J Chem Educ 64:561–567
Acknowledgements
Dr. K. Sharma thanks to the “Instituto de Investigaciones Biomédicas”, for the postdoctoral fellowship. The technical assistance of Mr. Ricardo Vázquez-Ramirez is highly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, K., Kubli-Garfias, C. Theoretical mechanism of the formation of cholesteryl chloride from cholesterol and thionyl chloride. J Mol Model 11, 135–140 (2005). https://doi.org/10.1007/s00894-004-0232-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-004-0232-7