Abstract
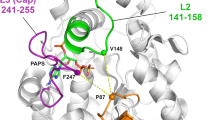
In order to understand the mechanisms of ligand binding and the interaction between the ligand and the bovine phenol sulfotransferase, (bSULT1A1, EC 2.8.2.1) a three-dimensional (3D) model of the bSULT1A1 is generated based on the crystal structure of the estrogen sulfotransferase (PDB code 1AQU) by using the InsightII/Homology module. With the aid of the molecular mechanics and molecular dynamics methods, the final refined model is obtained and is further assessed by Profile-3D and ProStat, which show that the refined model is reliable. With this model, a flexible docking study is performed and the results indicate that 3′-phosphoadenosine-5′- phosphosulfate (PAPS) is a more preferred ligand than coenzyme A (CoA), and that His108 forms hydrogen bond with PAPS, which is in good agreement with the experimental results. From these docking studies, we also suggest that Phe255, Phe24 and Tyr169 in bSULT1A1 are three important determinant residues in binding as they have strong van-der-Waals contacts with the ligand. The hydrogen–bonding interactions also play an important role for the stability of the complex. Our results may be helpful for further experimental investigations.
Figure The final 3D-structure of bSULT1A1. The structure is obtained by energy minimizing an average conformation over the last 100 ps of MD simulation. The α-helix is represented in red and the β-sheet in yellow.











Similar content being viewed by others
References
Roy AB (1981) Sulfation of drugs and related compounds. In: Mulder GJ (ed) CRC Press, Boca Raton, FL, pp 83–130
Matsui M, Homma H (1994) Int J Biochem 26:1237–1247
Falany CN, Wilborn TW (1994) Adv Pharmacol 27:301–329
Raftogianis RB, Wood C, Otterness DM, van Loon JA, Weinshilboum RM (1997) Biochem Biophys Res Commun 239:298–304
Wang Y, Spitz MR, Tsou AM-H, Zhang K, Makan N, Wu X (2002) Lung Cancer 35:137–142
Weinshilboum RM, Otterness DM (1994) Sulfotransferase enzymes. In: Kauffman FC (ed) Conjugation–deconjugation reactions in drug metabolism and toxicity. Chapter 22, “Handbook of experimental pharmacology” series, vol 112. Springer-Verlag, Berlin Heidelberg, pp 45–78
Falany CN (1991) Trends Pharmacol Sci 12:255–259
Leach M, Cameron E, Fite N, Stassinopoulos J, Palmreuter N, Beckmann JD (1999) Biochem Biophys Res Commun 261:815–819
Gamage NU, Duggleby RG, Barnett AC, Tresillian M, Latham CF, Liyou NE, McManus ME, Martin JL (2003) J Biol Chem 278:7655–7662
Otterness DM, Her C, Aksoy S, Kimura S, Wieben ED, Weinshilboum RM (1995) DNA Cell Biol 14:331–341
Her C, Aksoy IA, Kimura S, Brandriff BF, Wasmuth JJ, Weinshilboum RM (1995) Genomics 29:16–23
Kakuta Y, Negishi M, Pedersen LC (1997) Nat Struct Biol 4:904–908
InsightII, Version 98.0 (1998) Accelrys Inc. San Diego, USA
Homology User Guide (1999) Accelrys Inc. San Diego, USA
Lipman DJ, Pearson WR (1985) Science 227:1435–1441
Pearson WR, Lipman DJ (1988) Proc Natl Acad Sci USA 85:2444–2448
Pearson WR (1990) Meth Enzymol 183:63–98
Needleman SB, Wunch CD (1970) J Mol Biol 48:443–453
Bernstein FC, Koetzle TF, Williams GJB, Meyer EF Jr, Brice MD, Rodgers JR, Kennard O, Shimanouchi T, Tasumi M (1977) J Mol Biol 112:535–542
Discover3 User Guide (1999) Accelrys Inc. San Diego, USA
Luthy R, Bowie JU, Eisenberg D (1992) Nature 356:83–85
Profile-3D User Guide (1999) Accelrys Inc. San Diego, USA
Binding Site Analysis User Guide (1999) Accelrys Inc. San Diego, USA
Bidwell LM, Mcmanus ME, Gaedigk A, Kakuta Y, Negishi M, Pedersen JL, Martin L (1999) J Mol Biol 293:521–530
Li YF, Hata Y, Fujii T, Hisano T, Nishihara M, Kurihara T, Esaki N (1998) J Biol Chem 273:15035–15044
Affinity User Guide (1999) Accelrys Inc. San Diego, USA
Tulik GR, Chodavarapu S, Edgar R, Giannunzio L, Langland A, Schultz B, Beckmann JD (2002) J Biol Chem 277:39296–39303
Acknowledgements
This work was supported by the National Science Foundation of China (20333050, 20073014), Doctor Foundation by the Ministry of Education, Foundation for University Key Teacher by the Ministry of Education, Key subject of Science and Technology by the Ministry of Education of China, and Key subject of Science and Technology by Jilin Province.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, QC., Li, ZS., Xiao, JF. et al. Homology modeling and PAPS ligand (cofactor) binding study of bovine phenol sulfotransferase. J Mol Model 11, 97–104 (2005). https://doi.org/10.1007/s00894-004-0225-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-004-0225-6