Abstract
A homology model for the A2 domain of von Willebrand factor (VWF) is presented. A large number of target–template alignments were combined into a consensus alignment and used for constructing the model from the structures of six template proteins. Molecular dynamics simulation was used to study the structural and dynamic effects of eight mutations introduced into the model, all associated with type 2A von Willebrand disease. It was found that the group I mutations G1505R, L1540P and S1506L cause significant deviations over multiple regions of the protein, coupled to significant thermal fluctuations for G1505R and L1540P. This suggests that protein instability may be responsible for their intracellular retention. The group II mutations R1597W, E1638K and G1505E caused single loop displacements near the physiologic VWF proteolysis site between Y1605–M1606. These modest structural changes may affect interactions between VWF and the ADAMTS13 protease. The group II mutations I1628T and L1503Q caused no significant structural change in the protein, suggesting that inclusion of the protease in this model is necessary for understanding their effect.
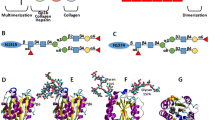
Figure Homology model of the von Willebrand factor A2 domain







Similar content being viewed by others
References
Sadler JE (1991) J Biol Chem 266:22777–22780
Ruggeri ZM, Ware J (1992) Thromb Haemost 67:594–599
Berliner S, Niiya K, Roberts JR, Houghten RA, Ruggeri ZM (1988) J Biol Chem 263:7500–7505
Voorberg J, Fontijn R, Calafat J, Janssen H, van Mourik JA, Pannekoek H (1991) J Cell Biol 113:195–205
Marti T, Rosselet SJ, Titani K, Walsh KA (1987) Biochemistry 26:8099–8109
Hoyer LW, Shainoff JR (1980) Blood 55:1056–1059
Federici AB, Bader R, Pagani S, Colibretti ML, De Marco L, Mannucci PM (1989) Br J Haematol 73:93–99
Schneppenheim R, Budde U, Oyen F, Angerhaus D, Aumann V, Drewke E, Hassenpflug W, Haberle J, Kentouche K, Kohne E, Kurnik K, Mueller-Wiefel D, Obser T, Santer R, Sykora K-W (2003) Blood 101:1845–1850
Kokame K, Matsumoto M, Soejima K, Yagi H, Ishizashi H, Funato M, Tamai H, Konno M, Kamide K, Kawano Y, Miyata T, Fujimura Y (2002) Proc Natl Acad Sci 99:11902–11907
Dent JA, Berkowitz SD, Ware J, Kasper CK, Ruggeri ZM (1990) Proc Natl Acad Sci 87:6306–6310
Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, Yang AY, Siemieniak DR, Stark KR, Gruppo R, Sarode R, Shurin SB, Chandrasekaran V, Stabler SP, Sabio H, Bouhassira EE, Upshaw Jr JD, Ginsburg D, Tsai HM (2001) Nature 413:488–494
Verweij CL, Quadt R, Briet E, Dubbeldam K, van Ommen GB, Pannekoek H (1988) J Clin Invest 81:1116–1121
Lyons SE, Cooney KA, Bockenstedt P, Ginsburg D (1994) Blood 83:1551–1557
Sadler JE (1994) Thromb Haemost 71:520–525
Lyons SE, Bruck ME, Bowie EJ, Ginsburg D (1992) J Biol Chem 267:4424–4430
Marti-Renom MA, Stuart AC, Fiser A, Sanchez R, Melo F, Sali A (2000) Annu Rev Biophys Biomol Struct 29:291–325
Jenkins PV, Pasi KJ, Perkins SJ (1998) Blood 91:2032–2044
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Nucleic Acids Res 25:3389–3402
Shindyalov IN, Bourne PE (1998) Protein Eng 11:739–747
Smith TF, Waterman MS (1981) J Mol Biol 147:195–197
Kelley LA, MacCallum RM, Sternberg MJ (2000) J Mol Biol 299:499–520
Fischer D (2000) World Sci 119–130
Jones DT (1999) J Mol Biol 287:797–815
Needleman SB, Wunsch CD (1970) J Mol Biol 48:443–453
MODELLER, version 6.0 (2001)http://www.salilab.org/modeller/modeller.html
Rychlewski L, Jaroszewski L, Li W, Godzik A (2000) Protein Sci 9:232–241
Bates P, Jackson RM, Sternberg MJ (1997) Proteins Suppl 1:59–67
Bates P, Kelley LA, MacCallum RM, Sternberg MJ (2001) Proteins Suppl 5:39–46
Karplus K, Barrett C, Hughey R (1998) Bioinformatics 14:846–856
Eddy SR (2001)http://hmmer.wustl.edu/
Bateman A, Birney E, Cerruti L, Durbin R, Etwiller L, Eddy SR, Griffiths-Jones S, Howe KL, Marshall M, Sonnhammer ELL (2002) Nucleic Acids Res 30:276–280
Grubmueller H (1996)http://www.mpibpc.gwdg.de/abteilungen/071/solvate/docu.html
Kale L, Skeel R, Bhandarkar M, Brunner R, Gursoy A, Krawetz N, Phillips J, Shinozaki A, Varadarajan K, Schulten K (1999) J Comput Phys 151:283–312
MacKerell AD, Bashford D, Bellott M, Dunbrack RL, Evanseck JD, Field MJ, Fischer S, Gao J, Guo H, Ha S, Joseph-McCarthy D, Kuchnir L, Kuczera K, Lau FTK, Mattos C, Michnick S, Ngo T, Nguyen DT, Prodhom B, Reiher III WE, Roux B, Schlenkrich M, Smith JC, Stote R, Straub J, Watanabe M, Wiorkiewicz-Kuczera J, Yin D, Karplus M (1998) J Phys Chem B 102:3586–3616
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) J Chem Phys 79:926–935
Ryckaert J-P, Ciccotti G, Berendsen HJC (1977) J Comput Phys 23:327–341
Darden T, York D, Pedersen L (1993) J Chem Phys 98:10089–10092
Feller SE, Zhang YH, Pastor RW, Brooks BR (1995) J Chem Phys 103:4613–4621
Tuckerman M, Berne BJ, Martyna GJ (1992) J Chem Phys 97:1990–2001
Lee B, Richards FM (1971) J Mol Biol 55:379–389
Kabsch W, Sander C (1983) Biopolymers 22:2577–2637
Lee JO, Bankston L, Arnaout MA, Liddington R (1995) Structure 15:1333–1340
Sauder JM, Arthur JW, Dunbrack RL (2000) Proteins 40:6–22
Jones DT (1999) J Mol Biol 292:195–202
Rost B, Sander C (1993) J Mol Biol 232:584–599
Cuff JA, Clamp ME, Siddiqui AS, Finlay M, Barton GJ (1998) Bioinformatics 14:892–893
Sippl MJ (1993) Proteins 17:355–362
Honig B, Nicholls A (1995) Science 268:1144–1149
Tramontano A, Leplae R, Morea V (2001) Proteins Suppl 5:22–38
Edwards YJ, Perkins SJ (1995) FEBS Lett 358:283–286
Edwards YJ, Perkins SJ (1996) J Mol Biol 260:277–285
Lehninger AL, Nelson DL, Cox MM (1993) Principles of biochemistry. Worth Publishers, New York
Lankhof H, Damas C, Schiphorst ME, Ijsseldijk MJW, Bracke M, Furlan M, de Groot PG, Sixma JJ, Vink T (1999) Thromb Haemost 81:976–983
Kokame K, Matsumoto M, Fujimura Y, Miyata T (2004) Blood 103:607–612
Acknowledgements
We thank Z. Jia and the Department of Biochemistry, and A. Becke for providing access to computing resources. JJS acknowledges support from a Canadian Institute of Health Research doctoral research award. LAO acknowledges support from a Canadian Blood Services graduate fellowship. DL is the recipient of a Canada Research Chair in Molecular Hemostasis and a Career Investigator Award from the Heart and Stroke Foundation of Ontario. DFW acknowledges support from the Canada Research Chairs program. This study has been funded in part by grants from the Canadian Institutes of Health Research (MOP42467) and the Heart and Stroke Foundation of Ontario (T4421).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Sutherland, J.J., O’Brien, L.A., Lillicrap, D. et al. Molecular modeling of the von Willebrand factor A2 Domain and the effects of associated type 2A von Willebrand disease mutations. J Mol Model 10, 259–270 (2004). https://doi.org/10.1007/s00894-004-0194-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-004-0194-9