Abstract
Intermolecular hydrogen-bond interactions in the monohydrated complexes of formamide, N-methylacetamide and glycylglycine have been studied using ab initio and DFT methods. The geometries were optimized using second-order Møller–Plesset perturbation theory and the B3LYP DFT functional with the 6-311++G** basis set. It is observed that hydrogen-bond interactions at the carbonyl group of the peptide moiety are stronger than those at the amino group of the formamide and N-methylacetamide molecules. Because of the presence of cyclic hydrogen-bonding interactions in glycylglycine, the interaction at the amino group is higher than at the carbonyl. The 13C and 15N NMR shielding values were calculated for the non-hydrated and monohydrated complexes. Condensed Fukui functions have also been calculated for non-hydrated formamide, N-methylacetamide and glycylglycine molecules at the B3LYP/6-311++G** level of theory, and the results are discussed.
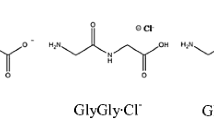
Figure Structure of hydrated glycylglycine dipeptide



Similar content being viewed by others
References
Jarrold ME (1999) Acc Chem Res 32:360–367
Daune M (1999) Molecular biophysics. Oxford University Press, Oxford
Lovas FJ, Suenram RD, Fraser GT (1988) J Chem Phys 88:722–729
Fraser GT, Suenram RD, Lovas FJ (1988) J Mol Struct 189:165–172
Engdahl A, Nelander B, Astrand PO (1993) J Chem Phys 99:4894–4907
Sieler G, Schweitzer-Stenner R (1997) J Am Chem Soc 119:1720–1726
Kameda T, Takeda N, Ando S, Ando I, Hashizume D, Ohashi Y (1998) Biopolymers 45:333–339
Manas ES, Getahun Z, Wright WW, de Grado WF, Vanderkooi JM (2000) J Am Chem Soc 122:9883–9890
Dixon DA, Dobbs KD, Valentini JJ (1994) J Phys Chem 98:13435–13439
Besley NA, Hirst JD (1999) J Am Chem Soc 121:8559–8566
Weir AF, Lowery AH, Williams RW (2001) Biopolymers 58:577–591
Nemukhin AV, Grigorenko BL, Bochenkova AV, Topol IA, Burt SK (2002) J Mol Struct (Theochem) 581:167–175
Fu A, Du D, Zhou Z (2003) J Mol Struct 623:315–325
Lecomte F, Lucas G, Gregoire G, Schermann JP, Desfrancois C (2002) Eur Phys J D 20:449-457
Tazaki K, Shimizu K (1998) J Phys Chem B 102:6419–6424
Apostolakis J, Ferrara P, Caflisch A (1999) J Chem Phys 110:2099–2108
Kalko SG, Guardia E, Padro JA (1999) J Phys Chem B 103:3935–3941
Abramov YA, Volkov A, Wu G, Coppens P (2000) J Phys Chem B 104:2183–2188
Chaudhuri P, Canuto S (2002) J Mol Struct (Theochem) 577:267–279
Møller C, Plesset MS (1934) Phys Rev 46:618–622
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Boys SF, Bernardi F (1970) Mol Phys 19:553–566
Sedano PS (2001) Doctoral dissertation. Universitat de Girona, Spain
Kulkarni SA, Bartolotti LJ, Pathak RK, (2003) Chem Phys Lett 372:620–626
Ditchfield R (1974) Mol Phys 27:789–807
Wolinski K, Hinton JF, Pulay P (1990) J Am Chem Soc 112:8251–8260
Yang W, Mortier WJ (1986) J Am Chem Soc 108:5708–5711
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery Jr JA, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels JD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Rega N, Salvador P, Dannenberg JJ, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (2001) Gaussian 98, Revision A.11.2. Gaussian, Pittsburgh, PA
Schaftenaar G, Noordik JH (2000) J Comput-Aided Mol Des 14:123–134
Scheiner S (1997) Hydrogen bonding: a theoretical perspective. Oxford University Press, New York
MacArthur MW, Thorton JM (1996) J Mol Biol 264:1180–1195
Senthilkumar K, Kolandaivel P (2002) Mol Phys 100:3817–3822
Basch H, Stevens WJ (1990) Chem Phys Lett 169:275–280
Acknowledgements
The authors thank Department of Science and Technology, Government of India, (DST) for financial assistance in the form of project (SP/S1/H-27/99) to complete this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Selvarengan, P., Kolandaivel, P.G. Molecular modeling of dipeptide and its analogous systems with water. J Mol Model 10, 198–203 (2004). https://doi.org/10.1007/s00894-004-0184-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-004-0184-y