Abstract
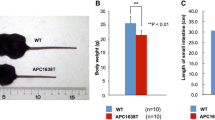
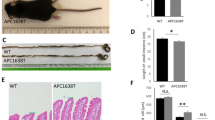
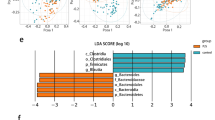
Adenomatous polyposis coli (APC) is recognized as an antioncogene related to familial adenomatous polyposis and colorectal cancers. However, APC is a large protein with multiple binding partners, indicating APC has diverse roles besides as a tumor suppressor. We have ever studied the roles of APC by using APC1638T/1638T (APC1638T) mice. Through those studies, we have noticed stools of APC1638T mice were smaller than those of APC+/+ mice and hypothesized there be a disturbance in fecal formation processes in APC1638T mice. The gut motility was morphologically analyzed by immunohistochemical staining of the Auerbach’s plexus. Gut microbiota was analyzed by terminal restriction fragment length polymorphism (T-RFLP). IgA concentration in stools was determined by enzyme-linked immunosorbent assay (ELISA). As results, macroscopic findings suggestive of large intestinal dysmotility and microscopic findings of disorganization and inflammation of the plexus were obtained in APC1638T mice. An alteration of microbiota composition, especially increased Bacteroidetes population was observed. Increases in IgA positive cells and dendritic cells in the ileum with high fecal IgA concentration were also confirmed, suggesting over-activation of gut immunity. Our findings will contribute to our understanding of APC’s functions in the gastrointestinal motility, and lead to a development of novel therapies for gut dysmotility-related diseases.





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D et al (1991) Identification of FAP locus genes from chromosome 5q21. Science 253(5020):661–665
Fodde R (2003) The multiple functions of tumour suppressors: it’s all in APC. Nat Cell Biol 5(3):190–192
Senda T, Iizuka-Kogo A, Onouchi T, Shimomura A (2007) Adenomatous polyposis coli (APC) plays multiple roles in the intestinal and colorectal epithelia. Med Mol Morphol 40(2):68–81
Smits R, Kielman MF, Breukel C, Zurcher C, Neufeld K, Jagmohan-Changur S, Hofland N, van Dijk J, White R, Edelmann W, Kucherlapati R, Khan PM, Fodde R (1999) Apc1638T: a mouse model delineating critical domains of the adenomatous polyposis coli protein involved in tumorigenesis and development. Genes Dev 13(10):1309–1321
Onouchi T, Kobayashi K, Sakai K, Shimomura A, Smits R, Sumi-Ichinose C, Kurosumi M, Takao K, Nomura R, Iizuka-Kogo A, Suzuki H, Kondo K, Akiyama T, Miyakawa T, Fodde R, Senda T (2014) Targeted deletion of the C-terminus of the mouse adenomatous polyposis coli tumor suppressor results in neurologic phenotypes related to schizophrenia. Mol Brain 7:21
Li C, Onouchi T, Hirayama M, Sakai K, Matsuda S, Yamada NO, Senda T (2020) Morphological and functional abnormalities of hippocampus in APC(1638T/1638T) mice. Med Mol Morphol 54(1):31–40
Wang T, Onouchi T, Yamada NO, Matsuda S, Senda T (2017) A disturbance of intestinal epithelial cell population and kinetics in APC1638T mice. Med Mol Morphol 50(2):94–102
Wenduerma YNO, Wang T, Senda T (2021) A further study on a disturbance of intestinal epithelial cell population and kinetics in APC1638T mice. Med Mol Morphol. 54:203–215
Yokoyama A, Nomura R, Kurosumi M, Shimomura A, Onouchi T, Iizuka-Kogo A, Smits R, Fodde R, Itoh M, Senda T (2012) Some fine-structural findings on the thyroid gland in Apc1638T/1638T mice that express a C-terminus lacking truncated Apc. Med Mol Morphol 45(3):161–167
Nakajima A, Sasaki T, Itoh K, Kitahara T, Takema Y, Hiramatsu K, Ishikawa D, Shibuya T, Kobayashi O, Osada T, Watanabe S, Nagahara A (2020) A soluble fiber diet increases bacteroides fragilis group abundance and immunoglobulin a production in the gut. Appl Environ Microbiol. https://doi.org/10.1128/AEM.00405-20
Yanagibashi T, Hosono A, Oyama A, Tsuda M, Hachimura S, Takahashi Y, Itoh K, Hirayama K, Takahashi K, Kaminogawa S (2009) Bacteroides induce higher IgA production than Lactobacillus by increasing activation-induced cytidine deaminase expression in B cells in murine Peyer’s patches. Biosci Biotechnol Biochem 73(2):372–377
Yanagibashi T, Hosono A, Oyama A, Tsuda M, Suzuki A, Hachimura S, Takahashi Y, Momose Y, Itoh K, Hirayama K, Takahashi K, Kaminogawa S (2013) IgA production in the large intestine is modulated by a different mechanism than in the small intestine: Bacteroides acidifaciens promotes IgA production in the large intestine by inducing germinal center formation and increasing the number of IgA+ B cells. Immunobiology 218(4):645–651
Nagashima K, Hisada T, Sato M, Mochizuki J (2003) Application of new primer-enzyme combinations to terminal restriction fragment length polymorphism profiling of bacterial populations in human feces. Appl Environ Microbiol 69(2):1251–1262
Baker GC, Smith JJ, Cowan DA (2003) Review and re-analysis of domain-specific 16S primers. J Microbiol Methods 55(3):541–555
Hayashi H, Sakamoto M, Benno Y (2004) Evaluation of three different forward primers by terminal restriction fragment length polymorphism analysis for determination of fecal bifidobacterium spp in healthy subjects. Microbiol Immunol 48(1):1–6
Phillips RJ, Hargrave SL, Rhodes BS, Zopf DA, Powley TL (2004) Quantification of neurons in the myenteric plexus: an evaluation of putative pan-neuronal markers. J Neurosci Methods 133(1–2):99–107
Bishop AE, Carlei F, Lee V, Trojanowski J, Marangos PJ, Dahl D, Polak JM (1985) Combined immunostaining of neurofilaments, neuron specific enolase, GFAP and S-100. A possible means for assessing the morphological and functional status of the enteric nervous system. Histochemistry 82(1):93–97
Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, Fanek Z, Liu L, Chen Z, Rothstein JD, Ransohoff RM, Gygi SP, Antel JP, Weiner HL (2014) Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci 17(1):131–143
Swanson MEV, Murray HC, Ryan B, Faull RLM, Dragunow M, Curtis MA (2020) Quantitative immunohistochemical analysis of myeloid cell marker expression in human cortex captures microglia heterogeneity with anatomical context. Sci Rep 10(1):11693
Koizumi T, Kerkhofs D, Mizuno T, Steinbusch HWM, Foulquier S (2019) Vessel-associated immune cells in cerebrovascular diseases: from perivascular macrophages to vessel-associated microglia. Front Neurosci 13:1291
Leyh J, Paeschke S, Mages B, Michalski D, Nowicki M, Bechmann I, Winter K (2021) Classification of microglial morphological phenotypes using machine learning. Front Cell Neurosci 15:701673
Kohrgruber N, Halanek N, Groger M, Winter D, Rappersberger K, Schmitt-Egenolf M, Stingl G, Maurer D (1999) Survival, maturation, and function of CD11c- and CD11c+ peripheral blood dendritic cells are differentially regulated by cytokines. J Immunol 163(6):3250–3259
Qualai J, Li LX, Cantero J, Tarrats A, Fernandez MA, Sumoy L, Rodolosse A, McSorley SJ, Genesca M (2016) Expression of CD11c Is associated with unconventional activated T cell subsets with high migratory potential. PLoS ONE 11(4):e0154253
Freytag C, Seeger J, Siegemund T, Grosche J, Grosche A, Freeman DE, Schusser GF, Hartig W (2008) Immunohistochemical characterization and quantitative analysis of neurons in the myenteric plexus of the equine intestine. Brain Res 1244:53–64
Lin Z, Gao N, Hu HZ, Liu S, Gao C, Kim G, Ren J, Xia Y, Peck OC, Wood JD (2002) Immunoreactivity of Hu proteins facilitates identification of myenteric neurones in guinea-pig small intestine. Neurogastroenterol Motil 14(2):197–204
Desmet AS, Cirillo C, Vanden BP (2014) Distinct subcellular localization of the neuronal marker HuC/D reveals hypoxia-induced damage in enteric neurons. Neurogastroenterol Motil 26(8):1131–1143
Swaminathan M, Kapur RP (2010) Counting myenteric ganglion cells in histologic sections: an empirical approach. Hum Pathol 41(8):1097–1108
Yntema CL, Hammond WS (1954) The origin of intrinsic ganglia of trunk viscera from vagal neural crest in the chick embryo. J Comp Neurol 101(2):515–541
Lake JI, Heuckeroth RO (2013) Enteric nervous system development: migration, differentiation, and disease. Am J Physiol Gastrointest Liver Physiol 305(1):G1-24
Tsai LH, Gleeson JG (2005) Nucleokinesis in neuronal migration. Neuron 46(3):383–388
Kapitein LC, Hoogenraad CC (2015) Building the neuronal microtubule cytoskeleton. Neuron 87(3):492–506
Bennett ML, Viaene AN (2021) What are activated and reactive glia and what is their role in neurodegeneration? Neurobiol Dis 148:105172
Hattori M, Taylor TD (2009) The human intestinal microbiome: a new frontier of human biology. DNA Res 16(1):1–12
Mabbott NA, Donaldson DS, Ohno H, Williams IR, Mahajan A (2013) Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol 6(4):666–677
Maynard CL, Elson CO, Hatton RD, Weaver CT (2012) Reciprocal interactions of the intestinal microbiota and immune system. Nature 489(7415):231–241
Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R (2016) Bowel disorders. Gastroenterology 150(6):1393–1407
Acknowledgements
We are grateful to Yutaka Hattori (Keyence, Osaka, Japan) for the helpful advice on using a Biorevo fluorescence microscopy BZ-X-800.
Funding
This work was supported by JSPS KAKENHI Grant Number JP15K08132 to Senda T.
Author information
Authors and Affiliations
Contributions
NOY designed the study. NOY and W performed the experiments. NOY wrote the manuscript. NOY, W, and TS contributed to data interpretation and manuscript revisions. TS supervised the study. all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The animal study proposal was approved by the Committee for Ethics in Animal Experimentation at Gifu University (permission number: 2019-004). All experiments were conducted in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yamada, N.O., Wenduerma & Senda, T. Altered microbiota caused by disordered gut motility leads to an overactivation of intestinal immune system in APC1638T mice. Med Mol Morphol 56, 177–186 (2023). https://doi.org/10.1007/s00795-023-00352-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-023-00352-1