Abstract
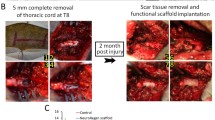
Traumatically injured spinal cord (SC) displays structural damage that includes discontinuity of long tracts and cavitations. Axonal regrowth beyond the lesion is necessary to achieve functional recovery following SC injury. We report here the development of an artificial collagen-filament (CF) scaffold to replace the SC in 8-week-old female Fisher rats. Axonal sprouting and regrowth was very rapid following grafting of the CF. One week after implantation, the scaffold was filled with cells of host origin and with regenerated axons. Histological examination of SC adjacent to the scaffold showed little cavity formation or fibrous scarring. Eight weeks after implantation, myelinated nerve fibers were found in the scaffold and 10–25 % of rubrospinal tracts were repaired. Four to six weeks after transplantation, motor evoked potentials were recorded in CF-grafted rats but were not detectable in non-grafted rats. Electrophysiological and histological examinations revealed the grafted CF was likely to function as a nerve tract. In addition, these results suggest that collagen fibers may provide a permissive microenvironment for the elongation of SC axons and to support the process of spinal cord regeneration.







Similar content being viewed by others
References
McDonald JW, Sadowsky C (2002) Spinal cord injury. Lancet 359:417–425
Suzuki H, Taguchi T, Tanaka H, Kataoka H, Li Z, Muramatsu K, Gondo T, Kawai S (2004) Neurospheres induced from bone marrow stromal cells are multipotent for differentiation into neuron, astrocyte, and oligodendrocyte phenotypes. Biochem Biophys Res Commun 322:918–922
Suzuki H, Taguchi T, Kato Y, Kanchiku T, Imagama T, Yara T, Moriya A, Muramatsu K, Tanaka T, Gondo T (2011) Transplantation of neurospheres derived from bone marrow stromal cells promotes neurological recovery in rats with spinal cord injury. Med Mol Morphol 44:131–138
Tator CH (2006) Review of treatment trials in human spinal cord injury: issues, difficulties, and recommendations. Neurosurgery 59:957–987
Rossignol S, Schwab M, Schwartz M, Fehlings MG (2007) Spinal cord injury: time to move? J Neuroscience 27:11782–11792
Onose G, Anghelescu A, Muresanu DF, Padure L, Haras MA, Chendreanu CO, Onose LV, Mirea A, Ciurea AV, El Masri WS, von Wild KR (2009) A review of published reports on neuroprotection in spinal cord injury. Spinal Cord 47:716–726
Chhabra HS, Lima C, Sachdeva S, Mittal A, Nigam V, Chaturvedi D, Arora M, Aggarwal A, Kapur R, Khan TA (2009) Autologous olfactory mucosal transplant in chronic spinal cord injury: an Indian Pilot Study. Spinal Cord 47:887–895
Stokic DS, Curt A (2010) Stem cells in the treatment of chronic spinal cord injury: evaluation of somatosensitive-evoked potentials in 39 patients. Spinal Cord 48:649
Wright KT, Masri WE, Osman A, Roberts S, Trivedi J, Ashton BA, Johnson WE (2008) The cell culture expansion of bone marrow stromal cells from humans with spinal cord injury: implications for future cell transplantation therapy. Spinal Cord 46:811–817
Yara T, Kato Y, Kataoka H, Kanchiku T, Suzuki H, Gondo T, Yoshii S, Taguchi T (2009) Environmental factors involved in axonal regeneration following spinal cord transection in rats. Med Mol Morphol 42:150–154
Tuszynski MH, Gabriel K, Gage FH, Suhr S, Meyer S, Rosetti A (1996) Nerve growth factor delivery by gene transfer induces differential outgrowth of sensory, motor, and noradrenergic neurites after adult spinal cord injury. Exp Neurol 137:157–173
Blesch A, Tuszynski MH (2003) Cellular GDNF delivery promotes growth of motor and dorsal column sensory axons after partial and complete spinal cord transections and induces remyelination. J Comp Neurol 467:403–417
Yoshii S, Oka M, Shima M, Akagi M, Taniguchi A (2003) Bridging a spinal cord defect using collagen filament. Spine 28:2346–2351
Yoshii S, Oka M, Shima M, Taniguchi A, Taki Y, Akagi M (2004) Restoration of function after spinal cord transection using a collagen bridge. J Biomed Mater Res 70:569–575
De la Torre JC, Gonzales-Carvajal M (1981) Steady state drug or fluid delivery to injured or transected spinal cord. Lab Anim Sci 31:701–703
Furutani T, Hino K, Okuda M, Gondo T, Nishina S, Kitase A, Korenaga M, Xiao SY, Weinman SA, Lemon SM, Sakaida I, Okita K (2006) Hepatic iron overload induces hepatocellular carcinoma in transgenic mice expressing the hepatitis C virus polyprotein. Gastroenterology 130:2087–2098
Kaneko K, Taguchi T, Morita H, Yonemura H, Fujimoto H, Kawai S (2001) Mechanism of prolonged central motor conduction time in compressive cervical myelopathy. Clin Neurophysiol 112:1035–1040
Wakabayashi Y, Komori H, Kawa-Uchi T, Mochida K, Takahashi M, Qi M, Otake K, Shinomiya K (2001) Functional recovery and regeneration of descending tracts in rats after spinal cord transection in infancy. Spine 26:1215–1222
Guo SZ, Ren XJ, Wu B, Jiang T (2010) Preparation of the acellular scaffold of the spinal cord and the study of biocompatibility. Spinal Cord 48:576–581
Geller M, Fawcett JW (2002) Building a bridge: engineering spinal cord repair. Exp Neurol 174:125–136
Jain A, Kim YT, McKeon RJ, Bellamkonda RV (2006) In situ gelling hydrogels for conformal repair of spinal cord defects, and local delivery of BDNF after spinal cord injury. Biomaterials 27:497–504
Huang YC, Huang YY (2006) Biomaterials and strategies for nerve regeneration. Artif Organs 30:514–522
Hurtado A, Moon LD, Maquet V, Blits B, Jérôme R, Oudega M (2006) Poly (d, l-lactic acid) macroporous guidance scaffolds seeded with Schwann cells genetically modified to secrete a bi-functional neurotrophin implanted in the completely transected adult rat thoracic spinal cord. Biomaterials 27:430–442
Moore MJ, Friedman JA, Lewellyn EB, Mantila SM, Krych AJ, Ameenuddin S, Knight AM, Lu L, Currier BL, Spinner RJ, Marsh RW, Windebank AJ, Yaszemski MJ (2006) Multiple-channel scaffolds to promote spinal cord axon regeneration. Biomaterials 27:419–429
Novikova LN, Novikov LN, Kellerth JO (2003) Biopolymers and biodegradable smart implants for tissue regeneration after spinal cord injury. Curr Opin Neurol 16:711–715
Novikova LN, Pettersson J, Brohlin M, Wiberg M (2008) Biodegradable poly-beta-hydroxybutyrate scaffold seeded with Schwann cells to promote spinal cord repair. Biomaterials 29:1198–1206
Tsai EC, Dalton PD, Shoichet MS, Tator CH (2006) Matrix inclusion within synthetic hydrogel guidance channels improves specific supraspinal and local axonal regeneration after complete spinal cord transection. Biomaterials 27:519–533
Friedman JA, Windebank AJ, Moore MJ, Spinner RJ, Currier BL, Yaszemski MJ (2002) Biodegradable polymer grafts for surgical repair of the injured spinal cord. Neurosurgery 51:742–751
Yamada H, Miyake T, Kitamura T (1995) Regeneration of axons in transection of the carp spinal cord. Zoolog Sci 12:325–332
Geisler S, Heilmann H, Veh RW (2001) An optimized method for simultaneous demonstration of neurons and myelinated fiber tracts for delineation of individual trunco- and palliothalamic nuclei in the mammalian brain. Histochem Cell Biol 117:69–79
Fujiki M, Kobayashi H, Inoue R, Ishii K (2004) Immediate plasticity in the motor pathways after spinal cord hemisection: implications for transcranial magnetic motor-evoked potentials. Exp Neurol 187:468–477
Cao Q, Zhang YP, Iannotti C, DeVries WH, Xu XM, Shields CB, Whittemore SR (2005) Functional and electrophysiological changes after graded traumatic spinal cord injury in adult rat. Exp Neurol 191:3–16
Ragnarsson KT (2008) Functional electrical stimulation after spinal cord injury: current use, therapeutic effects and future directions. Spinal Cord 46:255–274
Acknowledgments
We thank Jun Kawano (Kagoshima University, Kagoshima, Japan) for insightful discussions and for technical advice on the analysis of tracing study results. This work was supported by grants-in-aid for Young Scientific Research B (20791045) from the Ministry of Education, Science and Culture of Japan.
Conflict of interest
We declare that we have no conflict of interest regarding this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suzuki, H., Kanchiku, T., Imajo, Y. et al. Artificial collagen-filament scaffold promotes axon regeneration and long tract reconstruction in a rat model of spinal cord transection. Med Mol Morphol 48, 214–224 (2015). https://doi.org/10.1007/s00795-015-0104-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-015-0104-5