Abstract
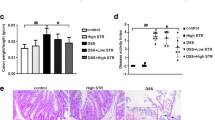
Intestinal fibrosis is a common and severe complication of inflammatory bowel disease (IBD), especially Crohn’s disease (CD). To investigate the therapeutic approach to intestinal fibrosis, we have developed a mouse model of intestinal fibrosis by administering dextran sulfate sodium (DSS) and examining the effects of irsogladine maleate (IM) [2,4-diamino-6-(2,5-dichlorophenyl)-s-triazine maleate], which has been widely used as an antiulcer drug for gastric mucosa in Japan, on DDS-induced chronic colitis. In this experimental colitis lesion, several pathognomonic changes were found: increased deposition of collagen, increased number of profibrogenic mesenchymal cells such as fibroblasts (vimentin+, α-SMA−) and myofibroblasts (vimentin+, α-SMA+) in both mucosa and submucosa of the colon with infiltrating inflammatory cells, and increased mRNA expressions of collagen type I, transforming growth factor (TGF)-β, matrix metalloproteinase (MMP)-2, and tissue inhibitor of matrix metalloproteinase (TIMP)-1. When IM was administered intrarectally to this colitis, all these pathological changes were significantly decreased or suppressed, suggesting a potential adjunctive therapy for intestinal fibrosis. IM could consequently reduce fibrosis in DSS colitis by direct or indirect effect on profibrogenic factors or fibroblasts. Therefore, the precise effect of IM on intestinal fibrosis should be investigated further.
Similar content being viewed by others
References
Podolsky DK (2002) Inflammatory bowel disease. N Engl J Med 347:417–429
Rutgeerts P, Vermeire S, Van Assche G (2009) Biological therapies for inflammatory bowel diseases. Gastroenterology 136:1182–1197
van Assche G, Geboes K, Rutgeerts P (2004) Medical therapy for Crohn’s disease strictures. Inflamm Bowel Dis 10:55–60
Regan MC, Flavin BM, Fitzpatrick JM, O’Connell PR (2000) Stricture formation in Crohn’s disease. The role of intestinal fibroblasts. Ann Surg 231:46–50
Yamamoto T (2005) Factors affecting recurrence after surgery for Crohn’s disease. World J Gastroenterol 11:3971–3979
Moskovitz DN, Assche GV, Maenhout B, Arts J, Ferrante M, Vermeire S, Rutgeerts P (2006) Incidence of colectomy during long-term follow-up after cyclosporine-induced remission of severe ulcerative colitis. Clin Gastroenterol Hepatol 4:760–765
Hiraishi H, Haruma K, Miwa H, Goto H (2010) Clinical trial: irsogladine maleate, a mucosal protective drug, accelerates gastric ulcer healing after treatment for eradication of Helicobacter pylori infection: the results of a multicentre, double-blind, randomized clinical trial (IMPACT study). Aliment Pharmacol Ther 31(8): 824–833
Murakami K, Okimoto T, Kodama M, Tanahashi J, Mizukami K, Shuto M, Abe H, Arita T, Fujioka T (2011) Comparison of the efficacy of irsogladine maleate and famotidine for the healing of gastric ulcers after Helicobacter pylori eradication therapy: a randomized, controlled, prospective study. Scand J Gastroenterol 46(3):287–292
Kyoi T, Oka M, Noda K, Ukai Y (2003) Irsogladine prevents monochloramine-induced gastric mucosal lesions by improving the decrease in mucosal blood flow due to the disturbance of nitric oxide synthesis in rats. J Pharmacol Sci 93:314–320
Tatsumi Y, Tanino M, Kodama T, Kashima K, Katsura M, Okua S (1998) Irsogladine maleate may preserve gastric mucosal hydrophobicity against ethanol in phospholipids independent way in rats. Jpn J Pharmacol 77:293–299
Kyoi T, Kitazawa S, Tajima K, Zhang X, Ukai Y (2004) Phosphodiesterase type IV inhibitors prevent ischemia-reperfusion-induced gastric injury in rats. J Pharmacol Sci 95:321–328
Zhang X, Tajima K, Kageyama K, Kyoi T (2008) Irsogladine maleate suppresses indomethacin-induced elevation of proinflammatory cytokines and gastric injury in rats. World J Gastroenterol 14:4784–4790
Ueda F, Kameda Y, Yamamoto O, Shibata Y (1994) Beta-adrenergic regulation of gap-junctional intercellular communication in cultured rabbit gastric epithelial cell. J Pharmacol Exp Ther 271:397–402
Sato M, Manabe N, Hata J, Ishii M, Kamada T, Kusunoki H, Shiotani A, Haruma K (2009) Effect of irsogladine maleate on NSAID-induced reduction of gastric mucosal blood flow in anesthetized dogs. Digestion 79:73–78
Kyoi T, Noda K, Oka M, Ukai Y (2004) Irsogladine, an anti-ulcer drug, suppresses superoxide production by inhibiting phosphodiesterase type 4 in human neutrophils. Life Sci 76:71–83
Kyoi T, Oka M, Noda K, Ukai Y (2004) Phosphodiesterase inhibition by a gastroprotective agent irsogladine: preferential blockade of cAMP hydrolysis. Life Sci 75:1833–1842
Kamei K, Kubo Y, Kato N, Hatazawa R, Aamgase K, Takeuchi K (2008) Prophylactic effects of irsogladine maleate against indomethacine-induced small intestinal lesions in rats. Dig Dig Sci 53:2657–2666
Fujita T, Kishimoto A, Shiba H, Hayashida K, Kajiya M, Uchida Y, Matsuda S, Takeda K, Ouhara K, Kawaguchi H, Abiko Y, Kurihara H (2010) Irsogladine maleate regulates neutrophil migration and E-cadherin expression in gingival epithelium stimulated by Aggregatibacter actinomycetemcomitans. Biochem Pharmacol 79(10):1496–1505
Macdonald TT (2003) A mouse model of intestinal fibrosis? Gastroenterology 125:1889–1892
McCormick BA (2008) Using Salmonella enterica serotype typhimurium to model intestinal fibrosis. Gastroenterology 134:872–875
Strober W, Fuss IJ, Blumberg RS (2002) The immunology of mucosal models of inflammation. Annu Rev Immunol 20:495–549
Melgar S, Karlsson A, Michaelsson E (2005) Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms. Am J Physiol Gastrointest Liver Physiol 288:G1328–G1338
Suzuki K, Sun X, Nagata M, Kawase T, Yamaguchi H, Sukumaran V, Kawauchi Y, Kawachi H, Nishino T, Watanabe K, Yoneyama H, Asakura H (2011) Analysis of intestinal fibrosis in chronic colitis in mice induced by dextran sulfate sodium. Pathol Int 61:228–238
Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R (1990) A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 98:694–702
Dielman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO (1994) Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology 107:1643–1652
Sun X, Suzuki K, Nagata M, Kawauchi Y, Yano M, Ohkoshi S, Matsuda Y, Kawachi H, Watanabe K, Asakura H, Aoyagi Y (2010) Rectal administration of tranilast ameliorated acute colitis in mice through increased expression of heme oxygenase-1. Pathol Int 60:93–101
Vallance BA, Gunawan MI, Hewlett B, Bercik P, Van Kampen C, Galeazzi F, Sime PJ, Gauldie J, Collins SM (2005) TGF-α1 gene transfer to the mouse colon leads to intestinal fibrosis. Am J Physiol Gastrointest Liver Physiol 289:G116–G128
Rieder F, Brenmoehl J, Leeb S, Scholmerich J, Rogler G (2007) Wound healing and fibrosis in intestinal disease. Gut 56:130–139
Macdonald TT (2003) A mouse model of intestinal fibrosis? Gastroenterology 125:1889–1892
McCormick BA (2008) Using Salmonella enterica serotype typhimurium to model intestinal fibrosis. Gastroenterology 134:872–875
Graham MF (1995) Pathogenesis of intestinal strictures in Crohn’s disease: an update. Inflamm Bowel Dis 1:220–227
Ueda F, Aratani S, Mimura K, Kimura K, Nomura A, Enomoto H (1984) Effect of 2,4-diamino-6-(2,5-dichlorophenyl)-s-triazine maleate (MN-1695) on gastric mucosal damage induced by various necrotizing agents in rats. Arzneim-Forsch 34:478–484
Ueda F, Ban K, Ishima T (1995) Irsogladine activates gap-junctional intercellular communication through M1 muscarinic acetylcholine receptor. J Pharmacol Exp Ther 274:815–819
Hara K, Ueda F (1996) Effect of irsogladine maleate on indomethacin-induced intestinal ulcers and trinitrobenzene sulfonic acidinduced colonic ulcers in rats. Jpn Pharmacol Ther 24:2143–2149
Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, Van Rees EP (1998) Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol 114:385–391
Motomura Y, Khan WI, El-Sharkawy RT, Verma-Gandhu M, Verdu EF, Gauldie J, Collins SM (2006) Induction of a fibrogenic response in mouse colon by overexpression of monocyte chemoattractant protein 1. Gut 55:662–670
Von Lampe B, Barthel B, Coupland SE, Riecken EO, Rosewicz S (2000) Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut 47:63–73
Martens MFWC, Huyben CMLC, Hendriks T (1992) Collagen synthesis in fibroblasts from human colon: regulatory aspects and differences with skin fibroblasts. Gut 33:1664–1670
Pucilowska JB, Williams KL, Lund PK (2000) Fibrogenesis IV. Fibrosis and inflammatory bowel disease: cellular mediators and animal models. Am J Physiol Gastrointest Liver Physiol 279:G653–G659
Tomasek JJ (2002) Myofibroblasts and mechano-regulation of connective tissue remodeling. Nat Rev Mol Cell Biol 3:349–363
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamaguchi, H., Suzuki, K., Nagata, M. et al. Irsogladine maleate ameliorates inflammation and fibrosis in mice with chronic colitis induced by dextran sulfate sodium. Med Mol Morphol 45, 140–151 (2012). https://doi.org/10.1007/s00795-011-0550-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-011-0550-7