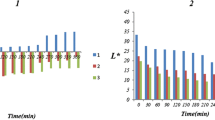
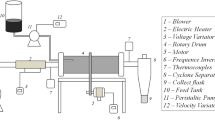
Abstract
Spray drying is appropriate for the preservation of halophilic microorganisms due to the nature of these microorganisms, as they survive in adverse environmental conditions by being encapsulated in salt crystals. Artificial neural networks were in this study used to optimize practically significant spray-drying regimes of the C50-carotenoids producer Halobacterium salinarum. Immediately after drying, the samples contained up to 54% halobacterial biomass and less than 5% moisture, and the level of preservation of carotenoids was 95–97%. The storage of biomass at 4 °C resulted in the gradual degradation of the carotenoids, which reached 58–64% in the best samples after 1 year. A comprehensive study of changes in halobacteria biomass after spray drying and the nature of the damage provided new data on the survival and preservation of cells and biologically active substances in the various spray-drying regimes and at different storage times.






Similar content being viewed by others
Abbreviations
- DMSO:
-
Dimethyl sulfoxide
- ANN:
-
Artificial neural network
- CFU:
-
Colony forming unit
References
Akolkar AV, Durai D, Desai AJ (2009) Halobacterium sp. SP1(1) as a starter culture for accelerating fish sauce fermentation. J Appl Microbiol 109:44–53. https://doi.org/10.1111/j.1365-2672.2009.04626.x
Ananta E, Volkert M, Knorr D (2005) Cellular injuries and storage stability of spray-dried Lactobacillus rhamnosus GG. Int Dairy J 15:399–409. https://doi.org/10.1016/j.idairyj.2004.08.004
Association of Official Analytical Chemists (2016) Official methods of analysis of AOAC international, 20th edn. AOAC Press, Gaithersburg
Baliga NS, Bjork SJ, Bonneau R, Pan M, Iloanusi C, Kottemann MC, Hood L, DiRuggiero J (2004) Systems level insights into the stress response to UV radiation in the halophilic archaeon Halobacterium NRC-1. Genome Res 14:1025–1035. https://doi.org/10.1101/gr.1993504
Behboudi-Jobbehdar S, Soukoulis C, Yonekura L, Fisk I (2013) Optimization of spray-drying process conditions for the production of maximally viable microencapsulated L. acidophilus NCIMB 701748. Dry Technol 31:1274–1283. https://doi.org/10.1080/07373937.2013.788509
DasSarma S, DasSarma P (2017) Halophiles. In: eLS. Wiley, Chichester, UK, pp 1–13. https://doi.org/10.1002/9780470015902.a0000394.pub4
DasSarma P, Coker JA, Huse V, DasSarma S (2010) Halophiles, industrial applications. In: Flickinger MC (ed) Encyclopedia of industrial biotechnology: bioprocess, bioseparation and cell technology. Wiley, Hoboken, NJ, pp 1–43
Desmond C, Stanton C, Fitzgerald GF, Collins K, Ross RP (2001) Environmental adaptation of probiotic lactobacilli towards improvement of performance during spray drying. Int Dairy J 11:801–808. https://doi.org/10.1016/S0958-6946(01)00121-2
Dummer AM, Bonsall JC, Cihla JB, Lawry SM, Johnson GC, Peck RF (2011) Bacterioopsin-mediated regulation of bacterioruberin biosynthesis in Halobacterium salinarum. J Bacteriol 193:5658–5667. https://doi.org/10.1128/JB.05376-11
El-Agamey A, Lowe GM, McGarvey DJ, Mortensen A, Phillip DM, Truscott TG, Young AJ (2004) Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch Biochem Biophys 430:37–48. https://doi.org/10.1016/j.abb.2004.03.007
Fendrihan S, Bérces A, Lammer H, Musso M, Rontó G, Polacsek TK, Holzinger A, Kolb C, Stan-Lotter H (2009) Investigating the effects of simulated Martian ultraviolet radiation on Halococcus dombrowskii and other extremely halophilic archaebacteria. Astrobiology 9:104–112. https://doi.org/10.1089/ast.2007.0234
Fu W-Y, Etzel MR (1995) Spray drying of Lactococcus lactis ssp. lactis C2 and cellular injury. J Food Sci 60:195–200. https://doi.org/10.1111/j.1365-2621.1995.tb05636.x
Ghandi A, Powell IB, Howes T, Chen XD, Adhikari B (2012) Effect of shear rate and oxygen stresses on the survival of Lactococcus lactis during the atomization and drying stages of spray drying: a laboratory and pilot scale study. J Food Eng 113:194–200. https://doi.org/10.1016/j.jfoodeng.2012.06.005
Goh F, Jeon YJ, Barrow K, Neilan BA, Burns BP (2011) Osmoadaptive strategies of the archaeon Halococcus hamelinensis isolated from a hypersaline stromatolite environment. Astrobiology 11:529–536. https://doi.org/10.1089/ast.2010.0591
Golowczyc MA, Silva J, Abraham AG, De Antoni GL, Teixeira P (2010) Preservation of probiotic strains isolated from kefir by spray drying. Lett Appl Microbiol 50:7–12. https://doi.org/10.1111/j.1472-765X.2009.02759.x
Gong P, Zhang L, Han X, Shigwedha N, Song W, Yi H, Du M, Cao C (2014) Injury mechanisms of lactic acid bacteria starter cultures during spray drying: a review. Drying Technol 32:793–800. https://doi.org/10.1080/07373937.2013.860458
Gruber C, Legat A, Pfaffenhuemer M, Radax C, Weidler G, Busse HJ, Stan-Lotter H (2004) Halobacterium noricense sp. nov., an archaeal isolate from a bore core of an alpine Permian salt deposit, classification of Halobacterium sp. NRC-1 as a strain of H. salinarum and emended description of H. salinarum. Extremophiles 8:431–439. https://doi.org/10.1007/s00792-004-0403-6
Jaakkola ST, Zerulla K, Guo Q, Liu Y, Ma H, Yang C, Bamford DH, Chen X, Soppa J, Oksanen HM (2014) Halophilic archaea cultivated from surface sterilized middle-late Eocene rock salt are polyploid. PLoS One 9:e110533. https://doi.org/10.1371/journal.pone.0110533
Kalenov SV, Baurina MM, Skladnev DA, Kuznetsov AY (2016) High-effective cultivation of Halobacterium salinarum providing with bacteriorhodopsin production under controlled stress. J Biotechnol 233:211–218. https://doi.org/10.1016/j.jbiotec.2016.07.014
Kochish II, Naidenskiy MS, Totoevva ME (2008) Efficacy of the immunostimulating Baxinum-vet preparation poultry (article in Russian). Poult Chick Prod 5:29–31
Kosanke JW, Osburn RM, Shuppe GI, Smith RS (1992) Slow rehydration improves the recovery of dried bacterial populations. Can J Microbiol 38:520–525. https://doi.org/10.1139/m92-086
Kottemann M, Kish A, Iloanusi C, Bjork S, DiRuggiero J (2005) Physiological responses of the halophilic archaeon Halobacterium sp. strain NRC1 to desiccation and gamma irradiation. Extremophiles 9:219–227. https://doi.org/10.1007/s00792-005-0437-4
Lavari L, Ianniello R, Páez R, Zotta T, Cuatrin A, Reinheimer J, Parente E, Vinderola G (2015) Growth of Lactobacillus rhamnosus 64 in whey permeate and study of the effect of mild stresses on survival to spray drying. LWT Food Sci Technol 63:322–330. https://doi.org/10.1016/j.lwt.2015.03.066
Leach G, Oliveira G, Morais R (1998) Spray-drying of Dunaliella salina to produce a β-carotene rich powder. J Ind Microbiol Biotechnol 20:82–85. https://doi.org/10.1038/sj.jim.2900485
López-Cortés A, Ochoa JL (1998) The biological significance of Halobacteria on nucleation and sodium chloride crystal growth. Stud Surf Sci Catal 120:903–923. https://doi.org/10.1016/S0167-2991(99)80384-X
Margesin R, Schinner F (2001) Potential of halotolerant and halophilic microorganisms for biotechnology. Extremophiles 5:73–83. https://doi.org/10.1007/s007920100184
Morgan CA, Herman N, White PA, Vesey G (2006) Preservation of micro-organisms by drying; a review. J Microbiol Methods 66:183–193. https://doi.org/10.1016/j.mimet.2006.02.017
Oren A (2002) Halophilic microorganisms and their environments. Kluwer Academic Publishers, Dordrecht, The Netherlands, p 575. https://doi.org/10.1007/0-306-48053-0
Oren A (2016) Life in hypersaline environments. In: Hurst CJ (ed) Their world: a diversity of microbial environments. Springer International Publishing, Cham, pp 301–339
Peighambardoust SH, Golshan Tafti A, Hesari J (2011) Application of spray drying for preservation of lactic acid starter cultures: a review. Trends Food Sci Technol 22:215–224. https://doi.org/10.1016/j.tifs.2011.01.009
Raposo MFJ, Morais AMMB, Morais RMSC (2012) Effects of spray-drying and storage on astaxanthin content of Haematococcus pluvialis biomass. World J Microbiol Biotechnol 28:1253–1257. https://doi.org/10.1007/s11274-011-0929-6
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Rodrigo-Baños M, Garbayo I, Vílchez C, Bonete MJ, Martínez-Espinosa RM (2015) Carotenoids from Haloarchaea and their potential in biotechnology. Mar Drugs 13:5508–5532. https://doi.org/10.3390/md13095508
Sakane T, Fukuda I, Itoh T, Yokota A (1992) Long-term preservation of halophilic archaebacteria and thermoacidophilic archaebacteria by liquid drying. J Microbiol Methods 16:281–287. https://doi.org/10.1016/0167-7012(92)90080-N
Salin ML, Brown-Peterson NJ (1993) Dealing with active oxygen intermediates: a halophilic perspective. Cell Mol Life Sci 49:523–529. https://doi.org/10.1007/BF01955155
Samborska K, Witrowa-Rajchert D, Gonçalves A (2005) Spray-drying of α-amylase—the effect of process variables on the enzyme inactivation. Dry Technol 23:941–953. https://doi.org/10.1081/DRT-200054243
Schuck P, Dolivet A, Méjean S, Hervé C, Jeantet R (2013) Spray drying of dairy bacteria: new opportunities to improve the viability of bacteria powders. Int Dairy J 31:12–17. https://doi.org/10.1016/j.idairyj.2012.01.006
Stan-Lotter H, Fendrihan S (2013) Survival strategies of halophilic oligotrophic and desiccation resistant prokaryotes. In: Seckbach J, Oren A, Stan-Lotter H (eds) Polyextremophiles. Life under multiple form of stress, Springer, Dordrecht, The Netherlands, pp 233–248. https://doi.org/10.1007/978-94-007-6488-0
Stan-Lotter H, Radax C, Gruber C, Legat A, Pfaffenhuemer M, Wieland H, Leuko S, Weidler G, Kömle N, Kargl G (2002) Astrobiology with haloarchaea from Permo-Triassic rock salt. Int J Astrobiol 1:S1473550403001307. https://doi.org/10.1017/S1473550403001307
Staroselov MA, Basova NY, Skhatum AK, Fedorov YuE, Pachina VV, Markov AN (2016) Effect of prebiotic Baxinum-vet on the intestinal microbiocenosis of newborn calves (article in Russian). Russ J Probl Vet Sanit Hyg Ecol 1:72–75
Teixeira P, Castro H, Kirby R (1995) Spray drying as a method for preparing concentrated cultures of Lactobacillus bulgaricus. J Appl Bacteriol 78:456–462. https://doi.org/10.1111/j.1365-2672.1995.tb03433.x
Teixeira P, Castro H, Kirby R (1996) Evidence of membrane lipid oxidation of spray-dried Lactobacillus bulgaricus during storage. Lett Appl Microbiol 22:34–38. https://doi.org/10.1111/j.1472-765X.1996.tb01103.x
Tindall BJ (1991) Cultivation and preservation of members of the family Halobacteriaceae. World J Microbiol Biotechnol 7:95–98. https://doi.org/10.1007/BF02310924
Vasil’ev IK (2007) Baxinum (book in Russian). Nikopharm, Moscow
Acknowledgements
The work is financially supported with the Grant of Russian Science Foundation No. 16-19-10469.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Oren.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kalenov, S.V., Gordienko, M.G., Murzina, E.D. et al. Halobacterium salinarum storage and rehydration after spray drying and optimization of the processes for preservation of carotenoids. Extremophiles 22, 511–523 (2018). https://doi.org/10.1007/s00792-018-1013-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-018-1013-z