Abstract
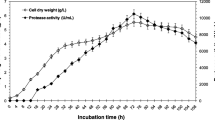
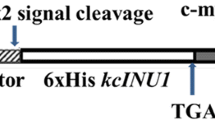
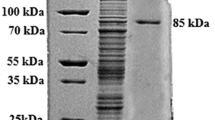
A glycoside hydrolase family 32 exo-inulinase gene was cloned from Sphingomonas sp. JB13 and expressed in Escherichia coli BL21 (DE3). The purified recombinant enzyme (rInuAJB13) showed an apparently optimal activity at pH 5.5 and 55 °C and remained activity at 10–70 °C. The addition of most metal ions and chemical reagents showed little or no effect (retaining more than 76.5 % activity) on the enzyme activity, notably the addition of surfactants SDS, CTAB, Tween 80, and Triton X-100. Most local liquid detergents, including Balin, Walch, Ariel, Tide, Tupperware, and Bluemoon, also showed little or no effect (retaining more than 77.8 % activity) on the enzyme activity. rInuAJB13 exhibited 135.3–163.6 % activity at the NaCl concentration of 1.0–4.5 M. After incubation with up to 57.0 mg mL−1 trypsin and 90.0 mg mL−1 proteinase K at 37 °C for 60 min (pH 7.2), rInuAJB13 retained more than 80 % of its initial activity. The enzyme presents a high proportion (28.0 %) of amino acid residues G, A, and V. This paper is the first to report a detergent-, salt-, and protease-tolerant exo-inulinase.




Similar content being viewed by others
References
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B (2009) The Carbohydrate-Active EnZymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res 37:D233–D238
Cao Y, Wang Y, Meng K, Bai Y, Shi P, Luo H, Yang P, Zhou Z, Zhang Z, Yao B (2009) A novel protease-resistant α-galactosidase with high hydrolytic activity from Gibberella sp. F75: gene cloning, expression, and enzymatic characterization. Appl Microbiol Biotechnol 83:875–884
Farias ME, Revale S, Mancini E, Ordonez O, Turjanski A, Cortez N, Vazquez MP (2011) Genome sequence of Sphingomonas sp. S17, isolated from an alkaline, hyperarsenic, and hypersaline volcano-associated lake at high altitude in the Argentinean Puna. J Bacteriol 193:3686–3687
Fontes C, Hall J, Hirst BH, Hazlewood GP, Gilbert HJ (1995) The resistance of cellulases and xylanases to proteolytic inactivation. Appl Microbiol Biotechnol 43:52–57
Ghazi S, Rooke JA, Galbraith H (2003) Improvement of the nutritive value of soybean meal by protease and α-galactosidase treatment in broiler cockerels and broiler chicks. Br Poult Sci 44:410–418
Gill PK, Manhas RK, Singh J, Singh P (2004) Purification and characterization of an exoinulinase from Aspergillus fumigatus. Appl Biochem Biotechnol 117:19–32
Gill PK, Manhas RK, Singh P (2006) Purification and properties of a heat-stable exoinulinase isoform from Aspergillus fumigatus. Bioresour Technol 97:894–902
Guo B, Chen XL, Sun CY, Zhou BC, Zhang YZ (2009) Gene cloning, expression and characterization of a new cold-active and salt-tolerant endo-β-1,4-xylanase from marine Glaciecola mesophila KMM 241. Appl Microbiol Biotechnol 84:1107–1115
Han YW (1990) Microbial Levan. Adv Appl Microbiol 35:171–194
Hung KS, Liu SM, Tzou WS, Lin FP, Pan CL, Fang TY, Sun KH, Tang SJ (2011) Characterization of a novel GH10 thermostable, halophilic xylanase from the marine bacterium Thermoanaerobacterium saccharolyticum NTOU1. Process Biochem 46:1257–1263
Jaswal SS, Sohl JL, Davis JH, Agard DA (2002) Energetic landscape of α-lytic protease optimizes longevity through kinetic stability. Nature 415:343–346
Kango N, Jain SC (2011) Production and properties of microbial inulinases: recent advances. Food Biotechnol 25:165–212
Kim KY, Koo BS, Jo D, Kim SI (2004) Cloning, expression, and purification of exoinulinase from Bacillus sp. snu-7. J Microbiol Biotechnol 14:344–349
Kobayashi T, Uchimura K, Deguchi S, Horikoshi K (2012) Cloning and sequencing of inulinase and β-fructofuranosidase genes of a deep-sea microbulbifer species and properties of recombinant enzymes. Appl Environ Microbiol 78:2493–2495
Kuddus M, Ramteke PW (2012) Recent developments in production and biotechnological applications of cold-active microbial proteases. Crit Rev Microbiol 38:330–338
Kushi RT, Monti R, Contiero J (2000) Production, purification and characterization of an extracellular inulinase from Kluyveromyces marxianus var. bulgaricus. J Ind Microbiol Biotechnol 25:63–69
Kwon YM, Kim HY, Choi YJ (2000) Cloning and characterization of Pseudomonas mucidolens exoinulinase. J Microbiol Biotechnol 10:238–243
Liebl W, Brem D, Gotschlich A (1998) Analysis of the gene for β-fructosidase (invertase, inulinase) of the hyperthermophilic bacterium Thermotoga maritima, and characterisation of the enzyme expressed in Escherichia coli. Appl Microbiol Biotechnol 50:55–64
Lima DM, Fernandes P, Nascimento DS, Ribeiro R, de Assis SA (2011) Fructose syrup: a biotechnology asset. Food Technol Biotechnol 49:424–434
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc 56:658–666
Madern D, Ebel C, Zaccai G (2000) Halophilic adaptation of enzymes. Extremophiles 4:91–98
Manning M, Colon W (2004) Structural basis of protein kinetic stability: resistance to sodium dodecyl sulfate suggests a central role for rigidity and a bias toward β-sheet structure. Biochemistry 43:11248–11254
Margesin R, Schinner F (2001) Potential of halotolerant and halophilic microorganisms for biotechnology. Extremophiles 5:73–83
Miller TR, Delcher AL, Salzberg SL, Saunders E, Detter JC, Halden RU (2010) Genome sequence of the dioxin-mineralizing bacterium Sphingomonas wittichii RW1. J Bacteriol 192:6101–6102
Morgavi DP, Beauchemin KA, Nsereko VL, Rode LM, McAllister TA, Iwaasa AD, Wang Y, Yang WZ (2001) Resistance of feed enzymes to proteolytic inactivation by rumen microorganisms and gastrointestinal proteases. J Anim Sci 79:1621–1630
Moriyama S, Akimoto H, Suetsugu N, Kawasaki S, Nakamura T, Ohta K (2002) Purification and properties of an extracellular exoinulinase from Penicillium sp. strain TN-88 and sequence analysis of the encoding gene. Biosci Biotechnol Biochem 66:1887–1896
Nagem RAP, Rojas AL, Golubev AM, Korneeva OS, Eneyskaya EV, Kulminskaya AA, Neustroev KN, Polikarpov I (2004) Crystal structure of exo-inulinase from Aspergillus awamori: the enzyme fold and structural determinants of substrate recognition. J Mol Biol 344:471–480
Prakash B, Vidyasagar M, Jayalakshmi SK, Sreeramulu K (2012) Purification and some properties of low-molecular-weight extreme halophilic xylanase from Chromohalobacter sp. TPSV 101. J Mol Catal B-Enzym 74:192–198
Sharma AD, Gill PK (2007) Purification and characterization of heat-stable exo-inulinase from Streptomyces sp. J Food Eng 79:1172–1178
Sheng J, Chi ZM, Gong F, Li J (2008) Purification and characterization of extracellular inulinase from a marine yeast Cryptococcus aureus G7a and inulin hydrolysis by the purified inulinase. Appl Biochem Biotechnol 144:111–121
Takahashi N, Mizuno F, Takamori K (1983) Isolation and properties of levanase from Streptococcus salivarius KTA-19. Infect Immun 42:231–236
Tsujimoto Y, Watanabe A, Nakano K, Watanabe K, Matsui H, Tsuji K, Tsukihara T, Suzuki Y (2003) Gene cloning, expression, and crystallization of a thermostable exo-inulinase from Geobacillus stearothermophilus KP1289. Appl Microbiol Biotechnol 62:180–185
Vijayalaxmi S, Prakash P, Jayalakshmi SK, Mulimani VH, Sreeramulu K (2013) Production of extremely alkaliphilic, halotolerent, detergent, and thermostable mannanase by the free and immobilized cells of Bacillus halodurans PPKS-2. Purification and characterization. Appl Biochem Biotechnol 171:382–395
Vijayaraghavan K, Yamini D, Ambika V, Sowdamini NS (2009) Trends in inulinase production-a review. Crit Rev Biotechnol 29:67–77
White DC, Sutton SD, Ringelberg DB (1996) The genus Sphingomonas: physiology and ecology. Curr Opin Biotechnol 7:301–306
Zhang LH, Zhao CX, Ohta WY, Wang YJ (2005) Inhibition of glucose on an exoinulinase from Kluyveromyces marxianus expressed in Pichia pastoris. Process Biochem 40:1541–1545
Zhou JP, Huang HQ, Meng K, Shi PJ, Wang YR, Luo HY, Yang PL, Bai YG, Yao B (2010) Cloning of a new xylanase gene from Streptomyces sp. TN119 using a modified thermal asymmetric interlaced-PCR specific for GC-rich genes and biochemical characterization. Appl Biochem Biotechnol 160:1277–1292
Zhou JP, Dong YY, Li JJ, Zhang R, Tang XH, Mu YL, Xu B, Wu Q, Huang ZX (2012) Cloning, heterologous expression, and characterization of novel protease-resistant α-galactosidase from new Sphingomonas strain. J Microbiol Biotechnol 22:1532–1539
Zhou JP, Gao YJ, Zhang R, Mo MH, Tang XH, Li JJ, Xu B, Ding JM, Huang ZX (2014) A novel low-temperature-active exo-inulinase identified based on Molecular-Activity strategy from Sphingobacterium sp. GN25 isolated from feces of Grus nigricollis. Process Biochem. doi:10.1016/j.procbio.2014.06.013
Acknowledgments
This work was supported by the Key Technologies Research and Development Program of China (No. 2013BAD10B01), National Natural Science Foundation of China (31260215), and Science Research Foundation of Yunnan Provincial Education Committee (2013Y432).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Oren.
J. Zhou and M. Peng contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, J., Peng, M., Zhang, R. et al. Characterization of Sphingomonas sp. JB13 exo-inulinase: a novel detergent-, salt-, and protease-tolerant exo-inulinase. Extremophiles 19, 383–393 (2015). https://doi.org/10.1007/s00792-014-0724-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-014-0724-z