Abstract
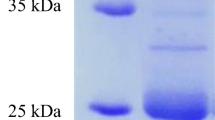
We isolated the feather-degrading Bacillus pseudofirmus FA30-01 from the soil sample of poultry farm. The isolate completely degraded feather pieces after liquid culture at 30°C (pH 10.5) for 3 days. Strain FA30-01 is a Gram-positive, spore-forming, rod-shaped bacterium and was identified with B. pseudofirmus based on 16S rDNA analysis. The keratinase enzyme produced by strain FA30-01 was refined using ammonium sulfate precipitation, negative-ion DEAE Toyopearl exchange chromatography, and hydroxyapatite chromatography. The refinement level was 14.5-fold. The molecular weight of this enzyme was 27.5 kDa and it had an isoelectric point of 5.9. The enzyme exhibited activity at pH 5.1–11.5 and 30–80°C with azokeratin as a substrate, although the optimum pH and temperature for keratinase activity were pH 8.8–10.3 and 60°C, respectively. This enzyme is one of the serine-type proteases. Subtilisin ALP I and this enzyme had 90% homology in the N-terminal amino acid sequence. Since this enzyme differed from ALP I in molecular weight, heat resistance and isoelectric point, they are suggested to be different enzymes.




Similar content being viewed by others
References
Anson ML (1938) The estimation of pepsin, trypsin, papain, and cathepsin with hemoglobin. J Gen Physiol 22:79–89
Atalo K, Gashe BA (1993) Protease production by a thermophilic Bacillus species (P-001A) which degrades various kinds of fibrous proteins. Biotechnol Lett 15:1151–1156
Böckle B, Galunsky B, Müller R (1995) Characterization of a keratinolytic serine proteinase from Streptomyces pactum DSM 40530. Appl Environ Microbiol 61:3705–3710
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bressollier P, Letourneau F, Urdaci M, Verneuil B (1999) Purification and characterization of a keratinolytic serine proteinase from Streptomyces albidoflavus. Appl Environ Microbiol 65:2570–2576
Chitte RR, Nalawade VK, Dey S (1999) Keratinolytic activity from the broth of a feather-degrading thermophilic Streptomyces thermoviolaeus strain SD8. Lett Appl Microbiol 28:131–136
De Toni CH, Richter MF, Chagas JR, Henriques JAP, Termignoni C (2002) Purification and characterization of an alkaline serine endopeptidase from a feather-degrading Xanthomonas maltophilia strain. Can J Microbiol 48:342–348
El-Naghy MA, El-Ktatny MS, Fadl-Allah EM, Nazeer WW (1998) Degradation of chicken feathers by Chrysosporium georgiae. Mycopathologia 143:77–84
Friedrich AB, Antranikian G (1996) Keratin degradation by Fervidobacterium pernnavorans, a novel thermophilic anaerobic species of the order Thermotogales. Appl Environ Microbiol 62:2875–2882
Hakamada Y, Kobayashi T, Hitomi J, Kawai S, Ito S (1994) Molecular cloning and nucleotide sequence of the gene for an alkaline protease from the alkalophilic Bacillus sp. KSM-K16. J Ferment Bioeng 78:105–108
Ignatova Z, Gousterova A, Spassov G, Nedkov P (1999) Isolation and partial characterisation of extracellular keratinase from a wool degrading thermophilic actinomycete strain Thermoactinomyces candidus. Can J Microbiol 45:217–222
Jacobs M, Eliasson M, Uhlen M, Flock JI (1985) Cloning, sequencing and expression of subtilisin Carlsberg from Bacillus licheniformis. Nucleic Acids Res 13:8913–8926
Letourneau F, Soussotte V, Bressollier P, Branland P, Verneuil B (1998) Keratinolytic activity of Streptomyces sp. S.K1-02: a new isolated strain. Lett Appl Microbiol 26:77–80
Lin X, Lee CG, Casale ES, Shin JCH (1992) Purification and characterization of a keratinase from a feather-degrading Bacillus licheniformis strain. Appl Environ Microbiol 58:3271–3275
Lin X, Kelemen DW, Miller ES, Shin JCH (1995) Nucleotide sequence and expression of kerA, the gene encoding a keratinolytic protease of Bacillus licheniformis PWD-1. Appl Environ Microbiol 61:1469–1474
Lin X, Inglis GD, Yanke LJ, Cheng K-J (1999) Selection and characterization of feather-degrading bacteria from canola meal compost. J Ind Microbiol Biotechnol 23:149–153
Nam GW, Lee DW, Lee HS, Lee NJ, Kim BC, Choe EA, Hwang JK, Suhartono MT, Pyun YR (2002) Native-feather degradation by Fervidobacterium islandicum AW-1, a newly isolated keratinase-producing thermophilic anaerobe. Arch Microbiol 178:538–547
Rao MB, Tanksale AM, Ghatge MS, Deshpande VV (1998) Molecular and biotechnological aspects of microbial protease. Microbiol Mol Biol Rev 62(3):597–635
Riessen S, Antranikian G (2001) Isolation of Thermoanaerobacter keratinophilus sp. nov., a novel thermophilic, anaerobic bacterium with keratinolytic activity. Extremophiles 5:399–408
Riffel A, Brandelli A (2002) Isolation and characterization of a feather-degrading bacterium from the poultry processing industry. J Ind Microbiol Biotechnol 29:255–258
Sangali S, Brandelli A (2000) Feather keratin hydrolysis by a Vibrio sp. strain kr2. J Appl Microbiol 89:735–743
Suntornsuk W, Suntornsuk L (2003) Feather degradation by Bacillus sp. FK 46 in submerged cultivation. Bioresour Technol 86:239–243
Takami H, Akiba T, Horikoshi K (1990) Characterization of an alkaline protease from Bacillus sp. no. AH-101. Appl Microbiol Biotechnol 33:519–523
Takami H, Kobayashi T, Aono R, Horikoshi K (1992) Molecular cloning, nucleotide sequence and expression of the structural gene for a thermostable alkaline protease from Bacillus sp. no. AH-101. Appl Microbiol Biotechnol 38:101–108
Tomarelli RM, Charney J, Harding ML (1949) The use of azoalbumin as a substrate in the colorimetric determination of peptic and tryptic activity. J Lab Clin Med 34:428–433
Tsuchida O, Yamagata Y, Ishizuka T, Arai T, Yamada J (1986) An alkaline proteinase of an alkalophilic Bacillus sp. Curr Microbiol 14:7–12
Varela H, Ferrari MD, Belobrajdic L, Vázquez A, Loperena ML (1997) Skin unhairing proteases of Bacillus subtilis: production and partial characterization. Biotechnol Lett 19:755–758
Wells JA, Ferrari E, Henner DJ, Estell DA, Chen EY (1983) Cloning, sequencing, and secretion of Bacillus amyloliquefaciens subtilisin in Bacillus subtilis. Nucleic Acids Res 11:7911–7925
Werlang PO, Brandelli A (2005) Characterization of a novel feather-degrading Bacillus sp. strain. Appl Biochem Biotechnol 120:71–79
Williams CM, Richter CS, Mackenzie JM Jr, Shin JCH (1990) Isolation, identification and characterization of a feather-degrading bacterium. Appl Environ Microbiol 56:1509–1515
Yamada Y, Sato T, Hanzawa S, Ichishima E (1995) The structure of subtilisin ALP I from Alkalophilic Bacillus sp. NKS-2. Curr Microbiol 30:201–209
Zaghloul TL, Al-Bahra M, Al-Azmeh H (1998) Isolation, identification and keratinolytic activity of several feather-degrading bacterial isolates. Appl Biochem Biotechnol 70–72:207–213
Acknowledgements
Grant support from the Matsumoto farm is appreciated. We are grateful to Ayami Hideshima, Extremobiosphere Research Center, JAMSTEC, and Hiroaki Minegishi, Graduate School of Engineering, Toyo University, for 16SrDNA analysis. And we are also very grateful to Izumi Yoshikawa, Extremobiosphere Research Center, JAMSTEC, for NH2-terminal amino acids sequence.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Antranikian
Rights and permissions
About this article
Cite this article
Kojima, M., Kanai, M., Tominaga, M. et al. Isolation and characterization of a feather-degrading enzyme from Bacillus pseudofirmus FA30-01. Extremophiles 10, 229–235 (2006). https://doi.org/10.1007/s00792-005-0491-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-005-0491-y