Abstract
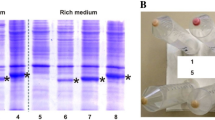
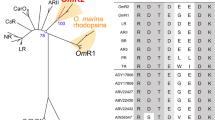
Pairs of PCR primers that targeted the archae/bacteriorhodopsin gene were used to clone the archaerhodopsin (aR) gene of Halorubrum xinjiangense strain BD-1T, and this gene was sequenced and functionally expressed in Escherichia coli. Recombinant E. coli cells harboring the plasmid carrying this gene became slightly purple or blue depending on whether they were supplemented with all- trans retinal or 3,4-dihydroretinal, respectively, during induction with IPTG. The purple and blue membranes from the recombinant E. coli showed maximal absorption at 555 and 588 nm, respectively, which are different from maximal absorption at 568 nm of the wild-type purple membrane. Purple membranes from the recombinant E. coli and from strain BD-1T were investigated in parallel. The E. coli purple membrane was fabricated into films and photoelectric responses were observed that depended on the light-on and light-off stimuli.



Similar content being viewed by others
References
Béjà O, Aravind L, Koonin EV, Suzuki MT, Hadd A, Nguyen LP, Jovanovich SB, Gates CM, Feldman RA, Spudich JL, Spudich EN, DeLong EF (2000) Bacterial rhodopsin: evidence for a new type of phototrophy in the sea. Science 289:1902–1906
Chu JF, Li XC, Zhang JP, Tang JA (2003) Fabrication and photoelectric response of ploly(allylamine hydrochloride)/PM thin films by layer-by-layer deposition technique. Biochem Biophys Res Commum 305:116–121
Corcelli A, Lattanzio VMT, Mascolo G, Papadia P, Fanizzi F (2002) Lipid-protein stoichiometries in a crystalline biological membrane: NMR quantitative analysis of the lipid extract of the purple membrane. J Lipid Res 43:132–140
Drachev LA, Drachev A, Chekulaeva LN, Evstigneeva RP, Kaulen AD, Khitrina LV, Khodonov AA, Lazarova ZR, Mitsner BI (1989) An investigation of the electrochemical cycle of bacteriorhodopsin analogs with the modified ring. Arch Biochem Biophys 270:184–197
Druzhko AB, Chamorovsky SK (1995) The cycle of photochromic reactions of a bacteriorhodopsin analog with 4-keto-retinal. Biosystems 35:133–136
Dunn RJ, Mccoy JM, Simsek M, Majumdar A, Chang SH, Rajbhandary UL, Khorana HG (1981) The bacteriorhodopsin gene. Proc Natl Acad Sci USA 78:6744–6748
Dunn RJ, Hackett NR, McCoy JM, Chao BH, Kimura K, Khorana HG (1987) Structure-function studies on bacteriorhodopsin. I. Expression of the bacterio-opsin gene in Escherichia coli. J Biol Chem 262(19):9246–9254
Feng J, Zhou P, Liu SJ (2004) Halorubrum xinjiangense, a novel halophile from saline lakes of China. Int J Syst Evol Microbiol 54:1789–1791
Frydrych M, Silfsten P, Parkkinen S, Parkkinen J, Jaaskelainen T (2000) Color sensitive retina based on bacteriorhodopsin. Biosystems 54:131–140
Hohenfeld IP, Wegener AA, Engelhard M (1999) Purification of histidine tagged bacteriorhodopsin, pharaonis halorhodopsin and pharaonis sensory rhodopsin II functionally expressed in Escherichia coli. FEBS Lett 442:198–202
Ihara K, Umemura T, Katagiri I, Kitajima-Ihara T, Sugiyama Y, Kimura Y, Mukohata Y (1999) Evolution of the archaeal rhodopsins: evolution rate changes by gene duplication and functional differentiation. J Mol Biol 285:163–174
Jussila T, Tkachenko NV, Parkkinen S, Lemmetyinen H (2001) Kinetics of photo-active bacteriorhodopsin analog 3,4-didehydroretinal. J Photochem Photobiol B 62:128–132
Khodonov AA, Demina OV, Khitrina LV, Kaulen AD, Silfsten P, Parkkinen S, Parkkinen J, Jaaskelainen T (1997) Modified bacteriorhodopsin as a basis for new optical devices. Sens Actuators B 38–39:218–221
Kitajima T, Hirayama J, Ihara K, Sugiyama Y, Kamo N, Mukohata Y (1996) Novel bacterial rhodopsins from Haloarcula vallismortis. Biochem Biophys Res Commum 220:341–345
Lukashev EP, Govindjee R, Kono M, Ebrey TG, Sugiyama Y, Mukohata Y (1994) pH dependence of the absorption spectra and photochemical transformations of the archaerhodopsins. Photochem Photobiol 60:69–75
Mukohata Y, Sugiyama Y, Ihara K, Yoshida M (1988) An Australian halobacterium contains a novel proton pump retinal protein: archaerhodopsin. Biochem Biophys Res Commu 151:1339–1345
Oesterhelt D, Stoeckenius W (1974) Isolation of the cell membrane of Halobacterium halobium and its function into red and purple membrane. In: Fleischer S, Packer L (eds) Methods in enzymology, vol 31, pp 667–678
Otomo J, Urabe Y, Tomioka H, Sasabe H (1992) The primary structures of helices A to G of three new bacteriorhodopsin-like retinal proteins. J Gen Microbiol 138:2389–2396
Sambrook J, Russell DW (2001) Molecular cloning, 3rd edn. CSHL Press, Cold Spring Harbor, New York
Shimono K, Iwamoto M, Sumi M, Kamo N (1997) Functional expression of pharaonis phoborhodopsin in Escherichia coli. FEBS Lett 420:54–56
Sugiyama Y, Maeda M, Futai M, Mukohata Y (1989) Isolation of a gene that encodes a new retinal protein, archaerhodopsin, from Halobacterium sp. aus-1. J Biol Chem 264:20859–20862
Tateno M, Ihara K, Mukohata Y (1994) The novel ion pump rhodopsins from Haloarcula form a family independent from both the bacteriorhodopsin and archaerhodopsin families/tribes. Arch Biochem Biophys 315:127–132
Uegaki K, Sugiyama Y, Mukohata Y (1991) Archaerhodopsin-2, from Halobacterium sp. aus-2 futher reveals essential amino acid residues for light-driven proton pumps. Arch Biochem Biophys 286:107–110
Weetall HH, Druzhko A, Lera AR, Alvarez R, Robertson B (2000) Measurement of proton release and uptake by analogs of bacteriorhodopsin. Bioelectrochemistry 51:27–33
Wise KJ, Gillespie NB, Stuart JA, Krebs MP, Birge RR (2002) Optimization of bacteriorhodopsin for bioelectronic devices. Trends Biotechnol 20:387–394
Yatsunami R, Kawakami T, Ohtani H, Nakamura S (1997) Primary structure of the novel bacterial rhodopsin from extremely halophilic archaeon Haloarcula japonica strain TR-1. Nucleic Acids Symp Ser 37:111–112
Acknowledgements
This work was supported by grants from Chinese Academy of Sciences (KJCX1-SW-07) and from the Ministry of Science and Technology (2004CB719600). Careful reading and constructive suggestions by Prof. Dr. J. K. Lanyi at University of California, Irvine is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. D. Grant
Rights and permissions
About this article
Cite this article
Feng, J., Liu, HC., Chu, JF. et al. Genetic cloning and functional expression in Escherichia coli of an archaerhodopsin gene from Halorubrum xinjiangense. Extremophiles 10, 29–33 (2006). https://doi.org/10.1007/s00792-005-0468-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-005-0468-x