Abstract
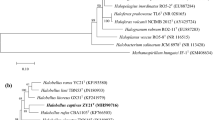
A novel extremely haloalkaliphilic, strictly anaerobic, acetogenic bacterium strain APO was isolated from sediments of the athalassic, meromictic, alkaline Mono Lake in California. The Gram-positive, spore-forming, slightly curved rods with sizes 0.55–0.7×1.7–3.0 μm were motile by a single laterally attached flagellum. Strain APO was mesophilic (range 10–48 °C, optimum of 37 °C); halophilic (NaCl range 1–20% (w/v) with optimum of 3–5% (w/v), and alkaliphilic (pH range 8.0–10.5, optimum 9.5). The novel isolate required sodium ions in the medium. Strain APO was an organotroph with a fermentative type of metabolism and used the substrates peptone, bacto-tryptone, casamino acid, yeast extract, l-serine, l-lysine, l-histidine, l-arginine, and pyruvate. The new isolate performed the Stickland reaction with the following amino acid pairs: proline + alanine, glycine + alanine, and tryptophan + valine. The main end product of growth was acetate. High activity of CO dehydrogenase and hydrogenase indicated the presence of a homoacetogenic, non-cycling acetyl-CoA pathway. Strain APO was resistant to kanamycin but sensitive to chloramphenicol, tetracycline, and gentamycin. The G+C content of the genomic DNA was 44.4 mol% (by HPLC method). The sequence of the 16S rRNA gene of strain APO possessed 98.2% similarity with the sequence from Tindallia magadiensis Z-7934, but the DNA-DNA hybridization value between these organisms was only 55%. On the basis of these physiological and molecular properties, strain APO is proposed to be a novel species of the genus Tindallia with the name Tindallia californiensis sp. nov., (type strain APO = ATCC BAA-393 = DSM 14871).



Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Ausubel FM, Brent R, Kingston RE, Moore DD, Smith JG, Sideman JG, Struhl K (eds) (1987) Current protocols in molecular biology. Wiley, New York, pp 2.10–2.11
De Ley J, Cattoir H, Reynaerts A (1970) The quantitative measurement of DNA hybridization from renaturation rates. Biochemistry 12:133–142
Gerhardt P, Murray RGE, Costilow RN, Nester EW, Wood WA, Krieg NR, Phillips GB (eds) (1981) Manual of methods for general bacteriology. American Society for Microbiology, Washington, DC
Gillis M, De Ley J, De Cleene M (1970) The determination of molecular weight of bacterial genome DNA from renaturation rates. Eur J Biochem 12:143–153
Grant WD, Tindall BJ (1986) The alkaline saline environment. In: Herbert RA, Codd GA (eds) Microbes in extreme environments. Academic, New York, pp 25–54
Hoover RB, Pikuta EV, Bej AK, Marsic D, Whitman WB, Tang J, Krader P (2003) Spirochaeta americana sp. nov., a new haloalkaliphilic, obligately anaerobic spirochete isolated from soda Mono Lake in California. Int J Syst Evol Microbiol 53:815–821
Johnson JL (1985) DNA reassociation and RNA hybridization of bacterial nucleic acids. Methods Microbiol 18:33–74
Jones BE, Grant WD, Duckworth AW, Owenson GG (1998) Microbial diversity of soda lakes. Extremophiles 2:191–200
Jukes TH, Cantor CR (1969) Evolution of protein molecules. In: Munro HM (ed) Mammalian protein metabolism. Academic, New York, pp 21–132
Kevbrin VV, Zhilina TN, Rainey FA, Zavarzin GA (1998) Tindallia magadii gen. nov., sp. nov.: an alkaliphilic anaerobic ammonifier from soda lake deposits. Curr Microbiol 37:94–100
Kumar S, Tamura K, Jakobsen IB, Nei M (2001) MEGA2: Molecular Evolutionary Genetics Analysis software. Bioinformatics 17:1244–1245
Lorowitz WH, Zhao H, Bryant MP (1989) Syntrophomonas wolfei subsp. saponavida subsp. nov., a long-chain fatty-acid-degrading, anaerobic, syntrophic bacterium; Syntrophomonas wolfei subsp. wolfei subsp. nov., and emended descriptions of the genus and species. Int J Syst Bacteriol 39:22–126
McInerney MJ, Bryant MP, Hespell RB, Costerton JW (1982) Syntrophomonas wolfei gen. nov., sp. nov., an anaerobic, syntrophic, fatty acid-oxidizing bacterium. Appl Environ Microbiol 41:1029–1039
Mesbah M, Premachandran U, Whitman WB (1989) Precise measurement of the G+C content of deoxyribonucleic acid by high-performance liquid chromatography. Int J Syst Bacteriol 39:159–167
Pikuta EV, Hoover RB (2001) Sulfate- and sulfur- reducing bacteria as terrestrial analogs for microbial life on Jupiter's satellite Io. In: Hoover RB, Levin GV, Paepe RR, Rozanov AYu (eds) Instruments, methods, and missions for astrobiology IV, vol. 4495. SPIE, San Diego, pp 232–254
Pikuta EV, Hoover RB, Bej AK, Marsic D, Whitman WB, Cleland D, Krader P (2003a) Desulfonatronum thiodismutans sp. nov., a new alkaliphilic sulfate-reducing bacterium capable of lithoautotrophic growth. Int J Syst Evol Microbiol (in press). http://dx.doi.org/10.1099/ijs.0.02598-0
Pikuta EV, Detkova EN, Bej AK, Marsic D, Hoover RB (2003b) Anaerobic halo-alkaliphilic bacterial community of athalassic, hypersaline Mono Lake and Owens Lake in California. In: Hoover RB, Rozanov A Yu, Paepe, RR (eds) Instruments, methods, and missions for Astrobiology VI, vol 4859. SPIE, Hawaii, pp 130–144
Pusheva MA, Berestovskaya YuYu, Borodulina NP (1989) The effect of nickel on the metabolism of homoacetogenic bacteria. Microbiology (Microbiologiya) 58:206–210
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Surkov AV, Dubinina GA, Lysenko AM, Glockner FO, Kuever J (2001) Dethiosulfovibrio russensis sp. nov., Dethiosulfovibrio marinus sp. nov. and Dethiosulfovibrio acidaminovorans sp. nov., novel anaerobic, thiosulfate- and sulfur- reducing bacteria isolated from "Thiodendron" sulfur mats in different saline environments. Int J Syst Evol Microbiol 51:327–337
Validation of the publication of new names and new combinations previously effectively published outside the IJSB. (1999) Int J Syst Bacteriol 49:1–3
Whitman WB, Ankwanda E, Wolfe RS (1982) Nutrition and carbon metabolism of Methanococcus voltae. J Bacteriol 149:852–863
Wolin EA, Wolin MJ, Wolfe RS (1963) Formation of methane by bacterial extracts. J Biol Chem 238:2882–2886
Zavarzin GA, Zhilina TN, Kevbrin VV (1999) The alkaliphilic microbial community and its functional diversity. Microbiology (Mikrobiologiya) 68:579–599
Zhilina TN, Zavarzin GA, Detkova EN, Rainey FA (1996) Natroniella acetigena gen. nov., sp. nov., an extremely haloalkalophilic, homoacetic bacterium: a new member of Haloanaerobiales. Curr Microbiol 32:320–326
Zhilina TN, Zavarzin GA, Rainey FA, Pikuta EV, Osipov GA, Kostrikina NA (1997) Desulfonatronovibrio hydrogenovorans gen. nov., sp. nov., an alkaliphilic, sulfate-reducing bacterium. Int J Syst Bacteriol 47:144–149
Zhilina TN, Detkova EN, Rainey FA, Osipov GA, Lysenko AM, Kostrikina NA, Zavarzin GA (1998) Natronoincola histidinovorans gen. nov., sp. nov., a new alkaliphilic acetogenic anaerobe. Curr Microbiol 37:177–185
Acknowledgements
We thank Dr. V. Kevbrin and Prof. J. Wiegel (University of Georgia in Athens) for their help with measuring end products, Prof. M. Farmer and Dr. J. Shields (Center for Advanced Ultrastructural Research of the University of Georgia in Athens) for Transmission Electron Microscopy and thin-section preparation. Also we are grateful to Dr. Jane Tang for organizing the analysis of fatty acids profile (ATCC), and Dr. John W. Shriver, Andrew T. Clark, William B. Peters (University of Alabama in Huntsville) for help with measuring hydrogenase and CO- dehydrogenase activity. We wish to acknowledge the NASA JSC Astrobiology Institute for Biomarkers in Astromaterials for supporting this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W.D. Grant
Rights and permissions
About this article
Cite this article
Pikuta, E.V., Hoover, R.B., Bej, A.K. et al. Tindallia californiensis sp. nov., a new anaerobic, haloalkaliphilic, spore-forming acetogen isolated from Mono Lake in California. Extremophiles 7, 327–334 (2003). https://doi.org/10.1007/s00792-003-0326-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-003-0326-7