Abstract
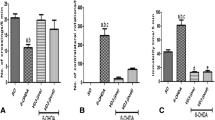
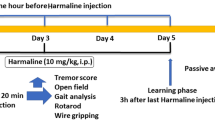
Children with chronic tic disorders (CTD), including Tourette syndrome (TS), have significantly reduced serum 25-hydroxyvitamin D [25(OH)D]. While vitamin D3 supplementation (VDS) may reduce tic symptoms in these children, its mechanism is unclear. The study aim was to investigate the effects and mechanisms of vitamin D deficiency (VDD) and VDS on TS model behavior. Forty 5-week-old male Sprague–Dawley rats were randomly divided into (n = 10 each): control, TS model, TS model with VDD (TS + VDD), or TS model with VDS (TS + VDS; two intramuscular injections of 20,000 IU/200 g) groups. The VDD model was diet-induced (0 IU vitamin D/kg); the TS model was iminodipropionitrile (IDPN)-induced. All groups were tested for behavior, serum and striatal 25(OH)D and dopamine (DA), mRNA expressions of vitamin D receptor (VDR), glial cell line-derived neurotrophic factor (GDNF), protooncogene tyrosine–protein kinase receptor Ret (c-Ret), and DA D1 (DRD1) and D2 (DRD2) receptor genes in the striatum. TS + VDD had higher behavior activity scores throughout, and higher total behavior score at day 21 compared with TS model. In contrast, day 21 TS + VDS stereotyped behavior scores and total scores were lower than TS model. The serum 25(OH)D in TS + VDD was < 20 ng/mL, and lower than control. Striatal DA of TS was lower than control. Compared with TS model, striatal DA of TS + VDD was lower, while in TS + VDS it was higher than TS model. Furthermore, mRNA expression of VDR, GDNF, and c-Ret genes decreased in TS model, and GDNF expression decreased more in TS + VDD, while TS + VDS had higher GDNF and c-Ret expressions. VDD aggravates, and VDS ameliorates tic-like behavior in an IDPN-induced model. VDS may upregulate GDNF/c-Ret signaling activity through VDR, reversing the striatal DA decrease and alleviating tic-like behavior.




Similar content being viewed by others
Data availability
The original data supporting the conclusions of this article will be made available on request.
Code availability
Not applicable.
Abbreviations
- CTDs:
-
Chronic tic disorders
- TS:
-
Tourette syndrome
- CSTC:
-
Cortico-striato-thalamo-cortical
- DRD2:
-
Dopamine D2 receptor
- DRD1:
-
Dopamine D1 receptor
- VD:
-
Vitamin D
- 25[OH]D:
-
25-Hydroxyvitamin D
- VDR:
-
Vitamin D receptor
- TH:
-
Enzyme–tyrosine hydroxylase
- GDNF:
-
Glial cell line-derived neurotrophic factor
- c-Ret:
-
Protooncogene tyrosine–protein kinase receptor Ret
- VDS:
-
Vitamin D3 supplementation
- VDD:
-
Vitamin D deficiency
- IDPN:
-
Iminodipropionitrile
- ELISA:
-
Enzyme-linked immunosorbent assay
- qPCR:
-
Quantitative real-time polymerase chain reaction
- SD:
-
Standard deviation
References
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Association, Washington
Jafari F, Abbasi P, Rahmati M, Hodhodi T, Kazeminia M (2022) Systematic review and meta-analysis of tourette syndrome prevalence; 1986 to 2022. Pediatr Neurol 137:6–16. https://doi.org/10.1016/j.pediatrneurol.2022.08.010
Scharf JM, Miller LL, Gauvin CA, Alabiso J, Mathews CA, Ben-Shlomo Y (2015) Population prevalence of Tourette syndrome: a systematic review and meta-analysis. Mov Disord 30:221–228. https://doi.org/10.1002/mds.26089
Szejko N, Robinson S, Hartmann A, Ganos C, Debes NM, Skov L, Haas M, Rizzo R, Stern J, Münchau A, Czernecki V, Dietrich A, Murphy TL, Martino D, Tarnok Z, Hedderly T, Müller-Vahl KR, Cath DC (2022) European clinical guidelines for Tourette syndrome and other tic disorders-version 2.0. Part I: assessment. Eur Child Adolesc Psychiatry 31:383–402. https://doi.org/10.1007/s00787-021-01842-2
Bloch MH, Leckman JF (2009) Clinical course of Tourette syndrome. J Psychosom Res 67:497–501. https://doi.org/10.1016/j.jpsychores.2009.09.002
Silvestri PR, Chiarotti F, Baglioni V, Neri V, Cardona F, Cavanna AE (2016) Health-related quality of life in patients with Gilles de la Tourette syndrome at the transition between adolescence and adulthood. Neurol Sci 37:1857–1860. https://doi.org/10.1007/s10072-016-2682-y
Vermilion J, Augustine E, Adams HR, Vierhile A, Lewin AB, Thatcher A, McDermott MP, O’Connor T, Kurlan R, van Wijngaarden E, Murphy TK, Mink JW (2020) Tic disorders are associated with lower child and parent quality of life and worse family functioning. Pediatr Neurol 105:48–54. https://doi.org/10.1016/j.pediatrneurol.2019.12.003
Aldred M, Cavanna AE (2015) Tourette syndrome and socioeconomic status. Neurol sci 36:1643–1649. https://doi.org/10.1007/s10072-015-2223-0
Abdulkadir M, Mathews CA, Scharf JM, Yu D, Tischfield JA, Heiman GA, Hoekstra PJ, Dietrich A (2019) Polygenic risk scores derived from a tourette syndrome genome-wide association study predict presence of tics in the avon longitudinal study of parents and children cohort. Biol Psychiat 85:298–304. https://doi.org/10.1016/j.biopsych.2018.09.011
Wendt FR, Pathak GA, Tylee DS, Goswami A, Polimanti R (2020) Heterogeneity and polygenicity in psychiatric disorders: a genome-wide perspective. Chronic stress (Thousand Oaks, Calif) 4:2470547020924844. https://doi.org/10.1177/2470547020924844
McCairn KW, Iriki A, Isoda M (2013) Global dysrhythmia of cerebro-basal ganglia-cerebellar networks underlies motor tics following striatal disinhibition. J Neurosci 33:697–708. https://doi.org/10.1523/jneurosci.4018-12.2013
Shitova AD, Zharikova TS, Kovaleva ON, Luchina AM, Aktemirov AS, Olsufieva AV, Sinelnikov MY, Pontes-Silva A, Zharikov YO (2023) Tourette Syndrome and Obsessive-Compulsive Disorder: A Comprehensive Review of Structural Alterations and Neurological Mechanisms. Behav Brain Res. https://doi.org/10.1016/j.bbr.2023.114606
Wen H, Liu Y, Wang J, Rekik I, Zhang J, Zhang Y, Tian H, Peng Y, He H (2016) Combining tract- and atlas-based analysis reveals microstructural abnormalities in early Tourette syndrome children. Hum Brain Mapp 37:1903–1919. https://doi.org/10.1002/hbm.23146
Leckman JF, Bloch MH, Smith ME, Larabi D, Hampson M (2010) Neurobiological substrates of Tourette’s disorder. J Child Adolesc Psychopharmacol 20:237–247. https://doi.org/10.1089/cap.2009.0118
Yael D, Vinner E, Bar-Gad I (2015) Pathophysiology of tic disorders. Mov disord 30:1171–1178. https://doi.org/10.1002/mds.26304
Wong DF, Brasić JR, Singer HS, Schretlen DJ, Kuwabara H, Zhou Y, Nandi A, Maris MA, Alexander M, Ye W, Rousset O, Kumar A, Szabo Z, Gjedde A, Grace AA (2008) Mechanisms of dopaminergic and serotonergic neurotransmission in Tourette syndrome: clues from an in vivo neurochemistry study with PET. Neuropsychopharmacol 33:1239–1251. https://doi.org/10.1038/sj.npp.1301528
Hartmann A, Worbe Y (2013) Pharmacological treatment of Gilles de la Tourette syndrome. Neurosci Biobehav Rev 37:1157–1161. https://doi.org/10.1016/j.neubiorev.2012.10.014
Bronfeld M, Israelashvili M, Bar-Gad I (2013) Pharmacological animal models of Tourette syndrome. Neurosci Biobehav Rev 37:1101–1119. https://doi.org/10.1016/j.neubiorev.2012.09.010
Zhang F, Li A (2015) Dual restoring effects of gastrodin on dopamine in rat models of Tourette’s syndrome. Neurosci Lett 588:62–66. https://doi.org/10.1016/j.neulet.2014.12.051
Turjanski N, Sawle GV, Playford ED, Weeks R, Lammerstma AA, Lees AJ, Brooks DJ (1994) PET studies of the presynaptic and postsynaptic dopaminergic system in Tourette’s syndrome. J Neurol Neurosurg Psychiatry 57:688–692. https://doi.org/10.1136/jnnp.57.6.688
Anca MH, Giladi N, Korczyn AD (2004) Ropinirole in Gilles de la Tourette syndrome. Neurology 62:1626–1627. https://doi.org/10.1212/01.wnl.0000123111.00324.bf
Black KJ, Mink JW (2000) Response to levodopa challenge in Tourette syndrome. Mov disord 15:1194–1198. https://doi.org/10.1002/1531-8257(200011)15:6%3c1194::aid-mds1019%3e3.0.co;2-h
Gilbert DL, Sethuraman G, Sine L, Peters S, Sallee FR (2000) Tourette’s syndrome improvement with pergolide in a randomized, double-blind, crossover trial. Neurol 54:1310–1315. https://doi.org/10.1212/wnl.54.6.1310
Eyles DW, Burne TH, McGrath JJ (2013) Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol 34:47–64. https://doi.org/10.1016/j.yfrne.2012.07.001
Scarmeas N, Anastasiou CA, Yannakoulia M (2018) Nutrition and prevention of cognitive impairment. The Lancet Neurol 17:1006–1015. https://doi.org/10.1016/s1474-4422(18)30338-7
Groves NJ, Burne THJ (2017) The impact of vitamin D deficiency on neurogenesis in the adult brain. Neural Regen Res 12:393–394. https://doi.org/10.4103/1673-5374.202936
Haindl MT, Üçal M, Wonisch W, Lang M, Nowakowska M, Adzemovic MZ, Khalil M, Enzinger C, Hochmeister S (2023) Vitamin d-an effective antioxidant in an animal model of progressive Multiple Sclerosis. Nutrients. https://doi.org/10.3390/nu15153309
Sassi F, Tamone C, D’Amelio P (2018) Vitamin d: nutrient, hormone, and immunomodulator. Nutrients. https://doi.org/10.3390/nu10111656
Liu Y, Li YW, Tang YL, Liu X, Jiang JH, Li QG, Yuan JY (2013) Vitamin D: preventive and therapeutic potential in Parkinson’s disease. Curr Drug Metab 14:989–993. https://doi.org/10.2174/1389200211314090005
Pertile RA, Cui X, Eyles DW (2016) Vitamin D signaling and the differentiation of developing dopamine systems. Neuroscience 333:193–203. https://doi.org/10.1016/j.neuroscience.2016.07.020
Cui X, Pertile R, Liu P, Eyles DW (2015) Vitamin D regulates tyrosine hydroxylase expression: N-cadherin a possible mediator. Neuroscience 304:90–100. https://doi.org/10.1016/j.neuroscience.2015.07.048
Hawes JE, Tesic D, Whitehouse AJ, Zosky GR, Smith JT, Wyrwoll CS (2015) Maternal vitamin D deficiency alters fetal brain development in the BALB/c mouse. Behav Brain Res 286:192–200. https://doi.org/10.1016/j.bbr.2015.03.008
Ibáñez CF, Andressoo JO (2017) Biology of GDNF and its receptors - Relevance for disorders of the central nervous system. Neurobiol Dis 97:80–89. https://doi.org/10.1016/j.nbd.2016.01.021
Pöyhönen S, Er S, Domanskyi A, Airavaara M (2019) Effects of neurotrophic factors in glial cells in the central nervous system: expression and properties in neurodegeneration and injury. Front Physiol 10:486. https://doi.org/10.3389/fphys.2019.00486
Pertile RAN, Cui X, Hammond L, Eyles DW (2018) Vitamin D regulation of GDNF/Ret signaling in dopaminergic neurons. Faseb J 32:819–828. https://doi.org/10.1096/fj.201700713R
Li HH, Shan L, Wang B, Du L, Xu ZD, Jia FY (2018) Serum 25-hyroxyvitamin D levels and tic severity in Chinese children with tic disorders. Psychiatry Res 267:80–84. https://doi.org/10.1016/j.psychres.2018.05.066
Li HH, Xu ZD, Wang B, Feng JY, Dong HY, Jia FY (2019) Clinical improvement following vitamin D3 supplementation in children with chronic tic disorders. Neuropsychiatr Dis Treat 15:2443–2450. https://doi.org/10.2147/ndt.S212322
Wang CX, Wang B, Sun JJ, Xiao CY, Ma H, Jia FY, Li HH (2023) Circulating retinol and 25(OH)D contents and their association with symptoms in children with chronic tic disorders. Eur Child Adolesc Psychiatry. https://doi.org/10.1007/s00787-023-02226-4
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:1911–1930. https://doi.org/10.1210/jc.2011-0385
Al Kadasah S, Al Mutairy A, Siddiquei M, Khan HA, Abdulwahid Arif I, Al Moutaery K, Tariq M (2009) Pentoxifylline attenuates iminodipropionitrile-induced behavioral abnormalities in rats. Behav Pharmacol 20:356–360. https://doi.org/10.1097/FBP.0b013e32832ec5ea
Diamond BI, Reyes MG, Borison R (1982) A new animal model for Tourette syndrome. Adv Neurol 35:221–225
Khan HA, Alhomida AS, Arif IA (2009) Neurovestibular toxicities of acrylonitrile and iminodipropionitrile in rats: a comparative evaluation of putative mechanisms and target sites. Toxicol sci 109:124–131. https://doi.org/10.1093/toxsci/kfp043
Lin L, Yu L, Xiang H, Hu X, Yuan X, Zhu H, Li H, Zhang H, Hou T, Cao J, Wu S, Su W, Li M (2019) Effects of acupuncture on behavioral stereotypies and brain dopamine system in mice as a model of tourette syndrome. Front Behav Neurosci 13:239. https://doi.org/10.3389/fnbeh.2019.00239
Cui X, Wang K, Zhang J, Cao ZB (2023) Aerobic exercise ameliorates myocardial fibrosis via affecting vitamin d receptor and transforming growth factor-β1 signaling in vitamin d-deficient mice. Nutrients. https://doi.org/10.3390/nu15030741
Yates NJ, Tesic D, Feindel KW, Smith JT, Clarke MW, Wale C, Crew RC, Wharfe MD, Whitehouse AJO, Wyrwoll CS (2018) Vitamin D is crucial for maternal care and offspring social behaviour in rats. J Endocrinol 237:73–85. https://doi.org/10.1530/joe-18-0008
Gogulothu R, Nagar D, Gopalakrishnan S, Garlapati VR, Kallamadi PR, Ismail A (2020) Disrupted expression of genes essential for skeletal muscle fibre integrity and energy metabolism in Vitamin D deficient rats. J Steroid Biochem Mol Biol 197:105525. https://doi.org/10.1016/j.jsbmb.2019.105525
Wang N, Wu X, Yang Q, Wang D, Wu Z, Wei Y, Cui J, Hong L, Xiong L, Qin D (2022) Qinglong zhidong decoction alleviated tourette syndrome in mice via modulating the level of neurotransmitters and the composition of gut microbiota. Front Pharmacol 13:819872. https://doi.org/10.3389/fphar.2022.819872
Chen J, Leong PK, Leung HY, Chan WM, Li Z, Qiu J, Ko KM, Chen J (2019) A Chinese herbal formulation, xiao-er-an-shen decoction, attenuates tourette syndrome, possibly by reversing abnormal changes in neurotransmitter levels and enhancing antioxidant status in mouse brain. Front Pharmacol 10:812. https://doi.org/10.3389/fphar.2019.00812
Wakata N, Araki Y, Sugimoto H, Iguchi H, Kinoshita M (2000) IDPN-induced monoamine and hydroxyl radical changes in the rat brain. Neurochem Res 25:401–404. https://doi.org/10.1023/a:1007553323461
Hirata H, Ogawa N, Asanuma M, Ota Z, Mori A (1993) Effect of chronic ceruletide treatment on dopaminergic neurotransmitters, receptors and their mRNAs in the striatum of rats with dyskinesia induced by iminodipropionitrile. Brain Res 604:197–204. https://doi.org/10.1016/0006-8993(93)90369-x
Ogawa N, Mizukawa K, Haba K, Sato H (1990) Neurotransmitter and receptor alterations in the rat persistent dyskinesia model induced by iminodipropionitrile. Eur Neurol 30(Suppl 1):31–40. https://doi.org/10.1159/000117171
Bond M, Moll N, Rosello A, Bond R, Schnell J, Burger B, Hoekstra PJ, Dietrich A, Schrag A, Kocovska E, Martino D, Mueller N, Schwarz M, Meier UC (2022) Vitamin D levels in children and adolescents with chronic tic disorders: a multicentre study. Eur Child Adolesc Psychiatry 31:1–12. https://doi.org/10.1007/s00787-021-01757-y
Xiaoxia L, Jilong J, Xianrui C, Yanhui C (2023) Vitamin D status and tic disorder: a systematic review and meta-analysis of observational studies. Front Pediatr 11:1173741. https://doi.org/10.3389/fped.2023.1173741
Greene DJ, Williams Iii AC, Koller JM, Schlaggar BL, Black KJ (2017) Brain structure in pediatric Tourette syndrome. Mol Psychiatry 22:972–980. https://doi.org/10.1038/mp.2016.194
Cui X, Gooch H, Groves NJ, Sah P, Burne TH, Eyles DW, McGrath JJ (2015) Vitamin D and the brain: key questions for future research. J Steroid Biochem Mol Biol 148:305–309. https://doi.org/10.1016/j.jsbmb.2014.11.004
Eyles D, Brown J, Mackay-Sim A, McGrath J, Feron F (2003) Vitamin D3 and brain development. Neuroscience 118:641–653. https://doi.org/10.1016/s0306-4522(03)00040-x
Almeras L, Eyles D, Benech P, Laffite D, Villard C, Patatian A, Boucraut J, Mackay-Sim A, McGrath J, Féron F (2007) Developmental vitamin D deficiency alters brain protein expression in the adult rat: implications for neuropsychiatric disorders. Proteomics 7:769–780. https://doi.org/10.1002/pmic.200600392
Frick L, Pittenger C (2016) Microglial dysregulation in ocd, tourette syndrome, and pandas. J Immunol Res 2016:8606057. https://doi.org/10.1155/2016/8606057
Spinello C, Laviola G, Macrì S (2016) Pediatric autoimmune disorders associated with streptococcal infections and Tourette’s syndrome in preclinical studies. Front Neurosci 10:310. https://doi.org/10.3389/fnins.2016.00310
Meza-Meza MR, Ruiz-Ballesteros AI, de la Cruz-Mosso U (2022) Functional effects of vitamin D: From nutrient to immunomodulator. Crit Rev Food Sci Nutr 62:3042–3062. https://doi.org/10.1080/10408398.2020.1862753
Verma R, Kim JY (2016) 1,25-Dihydroxyvitamin d3 facilitates m2 polarization and upregulates tlr10 expression on human microglial cells. NeuroImmunoModulation 23:75–80. https://doi.org/10.1159/000444300
Buse J, Schoenefeld K, Münchau A, Roessner V (2013) Neuromodulation in Tourette syndrome: dopamine and beyond. Neurosci Biobehav Rev 37:1069–1084. https://doi.org/10.1016/j.neubiorev.2012.10.004
Müller-Vahl KR, Loeber G, Kotsiari A, Müller-Engling L, Frieling H (2017) Gilles de la Tourette syndrome is associated with hypermethylation of the dopamine D2 receptor gene. J Psychiatr Res 86:1–8. https://doi.org/10.1016/j.jpsychires.2016.11.004
Trinko JR, Land BB, Solecki WB, Wickham RJ, Tellez LA, Maldonado-Aviles J, de Araujo IE, Addy NA, DiLeone RJ (2016) Vitamin d3: a role in dopamine circuit regulation, diet-induced obesity, and drug consumption. ENeuro. https://doi.org/10.1523/eneuro.0122-15.2016
Stumpf WE, Sar M, Clark SA, DeLuca HF (1982) Brain target sites for 1,25-dihydroxyvitamin D3. Science (New York, NY) 215:1403–1405. https://doi.org/10.1126/science.6977846
Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ (2005) Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat 29:21–30. https://doi.org/10.1016/j.jchemneu.2004.08.006
Cui X, Pelekanos M, Liu PY, Burne TH, McGrath JJ, Eyles DW (2013) The vitamin D receptor in dopamine neurons; its presence in human substantia nigra and its ontogenesis in rat midbrain. Neuroscience 236:77–87. https://doi.org/10.1016/j.neuroscience.2013.01.035
Prüfer K, Veenstra TD, Jirikowski GF, Kumar R (1999) Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J Chem Neuroanat 16:135–145. https://doi.org/10.1016/s0891-0618(99)00002-2
Kesby JP, Turner KM, Alexander S, Eyles DW, McGrath JJ, Burne THJ (2017) Developmental vitamin D deficiency alters multiple neurotransmitter systems in the neonatal rat brain. Int J Dev Neurosci 62:1–7. https://doi.org/10.1016/j.ijdevneu.2017.07.002
Kesby JP, Cui X, O’Loan J, McGrath JJ, Burne TH, Eyles DW (2010) Developmental vitamin D deficiency alters dopamine-mediated behaviors and dopamine transporter function in adult female rats. Psychopharmacology 208:159–168. https://doi.org/10.1007/s00213-009-1717-y
Zhu S, Zhao C, Wu Y, Yang Q, Shao A, Wang T, Wu J, Yin Y, Li Y, Hou J, Zhang X, Zhou G, Gu X, Wang X, Bustelo XR, Zhou J (2015) Identification of a Vav2-dependent mechanism for GDNF/Ret control of mesolimbic DAT trafficking. Nat Neurosci 18:1084–1093. https://doi.org/10.1038/nn.4060
Luan W, Hammond LA, Vuillermot S, Meyer U, Eyles DW (2018) Maternal Vitamin D Prevents Abnormal Dopaminergic Development and Function in a Mouse Model of Prenatal Immune Activation. Sci Rep 8:9741. https://doi.org/10.1038/s41598-018-28090-w
Sedaghat K, Yousefian Z, Vafaei AA, Rashidy-Pour A, Parsaei H, Khaleghian A, Choobdar S (2019) Mesolimbic dopamine system and its modulation by vitamin D in a chronic mild stress model of depression in the rat. Behav Brain Res 356:156–169. https://doi.org/10.1016/j.bbr.2018.08.020
Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F (1993) GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science (New York, NY) 260:1130–1132. https://doi.org/10.1126/science.8493557
Lin CI, Chang YC, Kao NJ, Lee WJ, Cross TW, Lin SH (2020) 1,25(OH)2D3 Alleviates aβ(25–35)-induced tau hyperphosphorylation, excessive reactive oxygen species, and apoptosis through interplay with glial cell line-derived neurotrophic factor signaling in sh-sy5y cells. Int J Mol Sci. https://doi.org/10.3390/ijms21124215
Naveilhan P, Neveu I, Wion D, Brachet P (1996) 1,25-Dihydroxyvitamin D3, an inducer of glial cell line-derived neurotrophic factor. NeuroReport 7:2171–2175. https://doi.org/10.1097/00001756-199609020-00023
Sanchez B, Lopez-Martin E, Segura C, Labandeira-Garcia JL, Perez-Fernandez R (2002) 1,25-Dihydroxyvitamin D(3) increases striatal GDNF mRNA and protein expression in adult rats. Brain Res Mol Brain Res 108:143–146. https://doi.org/10.1016/s0169-328x(02)00545-4
Orme RP, Bhangal MS, Fricker RA (2013) Calcitriol imparts neuroprotection in vitro to midbrain dopaminergic neurons by upregulating GDNF expression. PLoS ONE 8:e62040. https://doi.org/10.1371/journal.pone.0062040
Funding
Talent Reserve Program (TRP) of the First Hospital of Jilin University (JDYY-TRP-2024011) and the 2021 Pediatric Endocrinology Research Foundation for Young and Middle-aged Physicians (Z-2019–41-2101–01) sponsored this work.
Author information
Authors and Affiliations
Contributions
HL and FJ designed the research and provided funding; HL and XW drafted the manuscript; BW and XW participated in the process of this research and collected the data; HL and FJ contributed to the study concept and manuscript revision. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was carried out without any commercial or financial commitments that may become potential conflicts of interest.
Ethical approval
The study was approved by the Ethics Committee of Chongqing Western Biological Experimental Animal Ethics Committee (No: WST20220303S0401231[001]; Date of approval: March 3, 2022).
Informed consent
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors contributed to the article and approved the submitted version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, HH., Wang, XF., Wang, B. et al. Vitamin D3 improves iminodipropionitrile-induced tic-like behavior in rats through regulation of GDNF/c-Ret signaling activity. Eur Child Adolesc Psychiatry (2024). https://doi.org/10.1007/s00787-024-02376-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00787-024-02376-z