Abstract
Objective
Clinical trials and inconclusive meta-analyses have investigated the effects of omega-3 supplements in children with Attention-Deficit Hyperactivity Disorder (ADHD). We performed a randomised placebo-controlled trial to evaluate the efficacy of omega-3 fatty acids.
Methods
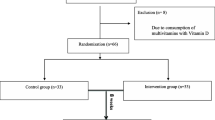
Children aged 6–15 years with established diagnosis of ADHD were randomised 1:1 to receive either supplements containing docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) or a placebo for 3 months. Psychotropic or omega-3-containing treatments were not authorised during the study. The primary outcome was the change in the Attention-Deficit Hyperactivity Disorder Rating Scale version 4 (ADHD-RS-IV). Other outcomes included safety, lexical level (Alouette test), attention (Test of Attentional Performance for Children—KiTAP), anxiety (48-item Conners Parent Rating Scale-Revised—CPRS-R), and depression (Children’s Depression Inventory—CDI).
Results
Between 2009 and 2011, 162 children were included in five French child psychiatry centres. The mean age was 9.90 (SD 2.62) years and 78.4% were boys. The inclusion ADHD-RS-IV at was 37.31 (SD 8.40). The total ADHD-RS-IV score reduction was greater in the placebo group than in the DHA–EPA group: −19 (−26, −12) % and −9.7 (−16.6, −2.9) %, respectively, p = 0.039. The other components of the Conners score had a similar variation but the differences between groups were not significant. Two patients in the DHA–EPA group and none in the placebo group experienced a severe adverse event (hospitalisation for worsening ADHD symptoms).
Conclusion
This study did not show any beneficial effect of omega-3 supplement in children with mild ADHD symptoms.

Similar content being viewed by others
References
APA (2010) Diagnostic and statistical manual of mental disorders, 4th edn, text revision (DSM-IV-TR). American Psychiatric Association. 943 pp
American Psychiatric Association (1996) DSM4 V, Manuel diagnostique et statistique des troubles mentaux. Traduction française, Paris, Masson, 1056 pp
Cantwell DP (1996) Attention deficit disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry 35(8):978–987
Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA (2007) The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 164(6):942–948
Faraone SV, Sergeant J, Gillberg C, Biederman J (2003) The worldwide prevalence of ADHD: is it an American condition? World Psychiatry 2(2):104–113
Schachter HM, Pham B, King J, Langford S, Moher D (2001) How efficacious and safe is short-acting methylphenidate for the treatment of attention-deficit disorder in children and adolescents? A meta-analysis. CAMJ 165(11):1475–1488
Attention deficit hyperactivity disorder: diagnosis and management. NICE guidelines [CG72] Published date: September 2008 Last updated: February 2016
Storebø OJ, Krogh HB, Ramstad E, Moreira-Maia CR, Holmskov M, Skoog M, Nilausen TD, Magnusson FL, Zwi M, Gillies D, Rosendal S, Groth C, Rasmussen KB, Gauci D, Kirubakaran R, Forsbøl B, Simonsen E, Gluud C (2015) Methylphenidate for attention-deficit/hyperactivity disorder in children and adolescents: cochrane systematic review with meta-analyses and trial sequential analyses of randomised clinical trials. BMJ 25(351):h5203
Kidwell KM, Van Dyk TR, Lundahl A, Nelson TD (2015) Stimulant medications and sleep for youth with ADHD: a meta-analysis. Pediatrics 136(6):1144–1153
Burgess JR, Stevens L, Zhang W, Peck L (2000) Long-chain polyunsaturated fatty acids in children with attention-deficit hyperactivity disorder. Am J Clin Nutr 71:327–330
Mitchell EA, Aman MG, Turbott SH, Manku M (1987) Clinical characteristics and serum essential fatty acid levels in hyperactive children. Clin Pediatr (Phila) 26(8):406–411
Burgess JR, Stevens L, Zhang W, Peck L (2000) Long-chain polyunsaturated fatty acids in children with attention-deficit hyperactivity disorder. Am J Clin Nutr 71(1 Suppl):327S–330S
Parletta N, Niyonsenga T, Duff J (2016) Omega-3 and omega-6 polyunsaturated fatty acid levels and correlations with symptoms in children with attention deficit hyperactivity disorder, autistic spectrum disorder and typically developing controls. PLoS ONE 11(5):e0156432
Richardson AJ, Puri BK (2000) The potential role of fatty acids in attention-deficit/hyperactivity disorder. Prostaglandins Leukot Essent Fatty Acids 63(1–2):79–87
Lange KW, Hauser J, Lange KM, Makulska-Gertruda E, Nakamura Y, Reissmann A, Sakaue Y, Takeuchi Y (2017) The role of nutritional supplements in the treatment of ADHD: what the evidence says. Curr Psychiatry Rep 19(2):8
Gillies D, Sinn JKh, Lad SS, Leach MJ, Ross MJ (2012) Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev, 7: p. CD007986
Sonuga-Barke EJ, Brandeis D, Cortese S, Daley D, Ferrin M, Holtmann M, Stevenson J, Danckaerts M, van der Oord S, Döpfner M, Dittmann RW, Simonoff E, Zuddas A, Banaschewski T, Buitelaar J, Coghill D, Hollis C, Konofal E, Lecendreux M, Wong IC, Sergeant J, European ADHD Guidelines Group (2013) Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry 170(3):275–289
Bloch MH, Qawasmi A (2011) Omega-3 fatty acid supplementation for the treatment of children with attention-deficit/hyperactivity disorder symptomatology: systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry 50(10):991–1000
DuPaul GJ, Power TJ, Anastopoulos AD (1998) ADHD Rating Scale IV: checklists, norms, and clinical interpretations. Guilford Press, New York (NY)
Mercier C, Roche S, Gaillard S et al (2016) Partial validation of a French version of the ADHD-rating scale IV on a French population of children with ADHD and epilepsy. Factorial structure, reliability, and responsiveness. Epilepsy Behav 58:1–6
Conners CK (1969) A teacher rating scale for use in drug studies with children. Am J Psychiatry 126(6):884–888
Lefavrais P. Test de l’Alouette, Éditions du Centre de Psychologie Appliquée. 2ème édition ed. 1967, Paris
Finch AJ Jr, Saylor CF, Edwards GL (1985) Children’s depression inventory: sex and grade norms for normal children. J Consult Clin Psychol 53(3):424–425
Michelson D, Allen AJ, Busner J et al (2002) Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry 159(11):1896–1901
Michelson D, Faries D, Wernicke J et al (2001) Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics 108(5):E83
Staquet Maurice J, Hays Ron D (1998) Peter M. Methods and Practice, Fayers. Quality of Life Assessment in Clinical Trials
Goodman D, Faraone SV, Adler LA et al (2010) Interpreting ADHD rating scale scores: linking ADHD rating scale scores and CGI levels in two randomized controlled trials of Lisdexamfetamine Dimesylate in ADHD. Primary Psychiatry 17(3):44–52
Hawkey E, Nigg JT (2014) Omega-3 fatty acid and ADHD: blood level analysis and meta-analytic extension of supplementation trials. Clin Psychol Rev 34(6):496–505
Stevens L, Zhang W, Peck L et al (2003) EFA supplementation in children with inattention, hyperactivity, and other disruptive behaviors. Lipids 38(10):1007–1021
Gustafsson PA, Birberg-Thornberg U, Duchén K et al (2010) EPA supplementation improves teacher-rated behaviour and oppositional symptoms in children with ADHD. Acta Paediatr 99(10):1540–1549
Itomura M, Hamazaki K, Sawazaki S et al (2005) The effect of fish oil on physical aggression in schoolchildren—a randomized, double-blind, placebo-controlled trial. J Nutr Biochem 16(3):163–171
Kirby A, Woodward A, Jackson S, Wang Y, Crawford MA (2010) A double-blind, placebo-controlled study investigating the effects of omega-3 supplementation in children aged 8–10 years from a mainstream school population. Res Dev Disabil 31(3):718–730
Richardson AJ, Burton JR, Sewell RP, Spreckelsen TF, Montgomery P (2012) Docosahexaenoic acid for reading, cognition and behavior in children aged 7–9 years: a randomized, controlled trial (the DOLAB Study). PLoS One 7(9):e43909. doi:10.1371/journal.pone.0043909. Epub 2012 Sep 6
Bos DJ, van Montfort SJ, Oranje B, Durston S, Smeets PA (2016) Effects of omega-3 polyunsaturated fatty acids on human brain morphology and function: what is the evidence? Eur Neuropsychopharmacol 26(3):546–561. doi:10.1016/j.euroneuro.2015.12.031. Epub 2015 Dec 21. Review
Vyncke KE, Libuda L, De Vriendt T, Moreno LA, Van Winckel M, Manios Y, Gottrand F, Molnar D, Vanaelst B, Sjöström M, González-Gross M, Censi L, Widhalm K, Michels N, Gilbert CC, Xatzis C, Cuenca García M, de Heredia FP, De Henauw S, Huybrechts I; HELENA consortium (2012) Dietary fatty acid intake, its food sources and determinants in European adolescents: the HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) Study. Br J Nutr 108(12):2261–273. doi:10.1017/S000711451200030X. Epub 2012 Feb 28
Koletzko B, Uauy R, Palou A, Kok F, Hornstra G, Eilander A, Moretti D, Osendarp S, Zock P, Innis S (2010) Dietary intake of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in children—a workshop report. Br J Nutr 103(6):923–928
Voigt RG, Llorente AM, Jensen CL, Fraley JK, Berretta MC, Heird WC (2001) A randomized, double-blind, placebo-controlled trial of docosahexaenoic acid supplementation in children with attention-deficit/hyperactivity disorder. J Pediatr 139(2):189–196
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare they have no conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Cornu, C., Mercier, C., Ginhoux, T. et al. A double-blind placebo-controlled randomised trial of omega-3 supplementation in children with moderate ADHD symptoms. Eur Child Adolesc Psychiatry 27, 377–384 (2018). https://doi.org/10.1007/s00787-017-1058-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00787-017-1058-z