Abstract
Lisdexamfetamine dimesylate (LDX) is a long-acting, prodrug stimulant therapy for patients with attention-deficit/hyperactivity disorder (ADHD). This randomized placebo-controlled trial of an optimized daily dose of LDX (30, 50 or 70 mg) was conducted in children and adolescents (aged 6–17 years) with ADHD. To evaluate the efficacy of LDX throughout the day, symptoms and behaviors of ADHD were evaluated using an abbreviated version of the Conners’ Parent Rating Scale-Revised (CPRS-R) at 1000, 1400 and 1800 hours following early morning dosing (0700 hours). Osmotic-release oral system methylphenidate (OROS-MPH) was included as a reference treatment, but the study was not designed to support a statistical comparison between LDX and OROS-MPH. The full analysis set comprised 317 patients (LDX, n = 104; placebo, n = 106; OROS-MPH, n = 107). At baseline, CPRS-R total scores were similar across treatment groups. At endpoint, differences (active treatment − placebo) in least squares (LS) mean change from baseline CPRS-R total scores were statistically significant (P < 0.001) throughout the day for LDX (effect sizes: 1000 hours, 1.42; 1400 hours, 1.41; 1800 hours, 1.30) and OROS-MPH (effect sizes: 1000 hours, 1.04; 1400 hours, 0.98; 1800 hours, 0.92). Differences in LS mean change from baseline to endpoint were statistically significant (P < 0.001) for both active treatments in all four subscales of the CPRS-R (ADHD index, oppositional, hyperactivity and cognitive). In conclusion, improvements relative to placebo in ADHD-related symptoms and behaviors in children and adolescents receiving a single morning dose of LDX or OROS-MPH were maintained throughout the day and were ongoing at the last measurement in the evening (1800 hours).
Similar content being viewed by others
Introduction
Attention-deficit/hyperactivity disorder (ADHD) has an estimated worldwide prevalence among children of 5.3 % [1] and persists into adulthood in approximately two-thirds of patients [2–4]. It is a well-defined disorder that is characterized by persistent symptoms of hyperactivity, impulsivity and/or inattention, which are associated with serious impairments in academic, social and interpersonal functioning [5, 6]. Stimulant medications are commonly recommended as part of a comprehensive, multimodal treatment plan for patients with ADHD that also includes behavioral, psychoeducational and psychological interventions [7–10].
Lisdexamfetamine dimesylate (LDX) is the first chemically formulated, long-acting, prodrug stimulant; the active metabolite d-amfetamine is released enzymatically from the parent molecule in the blood [11]. In clinical trials conducted in the USA, LDX has been shown to be an effective once-daily treatment for ADHD in children, adolescents and adults [12–14]. The present study reports secondary efficacy outcomes from the first European phase 3 clinical trial of LDX (SPD489-325). Study SPD489-325 was a randomized, double-blind, placebo-controlled trial that evaluated the efficacy, safety and tolerability of dose-optimized LDX over the course of 7 weeks in children and adolescents aged 6–17 years with a diagnosis of ADHD that was of at least moderate severity [15]. Compared with placebo, the mean ADHD Rating Scale version IV (ADHD-RS-IV) total score in patients treated with LDX was significantly reduced from baseline to endpoint (effect size 1.80), and 78 % of individuals receiving LDX demonstrated clinically relevant improvements in Clinical Global Impressions-Improvement (CGI-I) scores. Patients receiving osmotic-release oral system methylphenidate (OROS-MPH, included as a reference arm in this study) also showed significant improvements in ADHD-RS-IV total score (effect size 1.26) and CGI-I scores versus placebo. Both active treatments were well tolerated.
The duration of therapeutic effect is an important consideration for clinicians when developing individualized treatment plans for patients with ADHD. In North American study populations, the beneficial effects of LDX treatment were reported to be ongoing at the last daily time-point assessed (13 h post-dose in children and 14 h post-dose in adults) [16, 17]. Here, we examine whether the effects of LDX are maintained throughout the day (3, 7 or 11 h post-dose) in a European study of children and adolescents with ADHD, using an abbreviated version of the Conners’ Parent Rating Scale-Revised (CPRS-R) to evaluate ADHD-related symptoms and problem behaviors.
Methods
Patients and study design
The study protocol (ClinicalTrials.gov Identifier: NCT00763971) was approved by an independent ethics committee/institutional review board and regulatory agency in each center (as appropriate). The study was conducted in accordance with current international and local applicable regulations, and written informed consent was obtained from each participant or legally appointed representative.
The design of this randomized, double-blind, parallel-group, dose-optimized, placebo-controlled study has been described in detail previously [15]. The study included a reference arm comprising patients treated with an optimized dose of OROS-MPH, but the study was not powered to make inferential statistical comparisons between LDX and OROS-MPH. Male and female children and adolescents (aged 6–17 years) who satisfied the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision criteria [6] for a primary diagnosis of ADHD were enrolled. Eligible patients had ADHD of at least moderate severity (ADHD-RS-IV total score of 28 or higher). The key exclusion criteria included failure to respond, based on the investigators’ judgement, to an adequate course (dose and duration) of OROS-MPH therapy and patients with a documented allergy, hypersensitivity or intolerance to amfetamine or methylphenidate. Individuals with a comorbid psychiatric diagnosis with significant symptoms (with the exception of oppositional defiant disorder) and those with conduct disorder were excluded. Patients whose current ADHD medication provided effective control of symptoms with acceptable tolerability were also excluded.
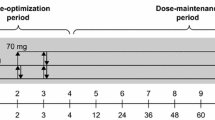
Eligible patients completed a screening and washout period (3–42 days) and were randomized in a 1:1:1 ratio at baseline (visit 0) to receive once-daily LDX (30, 50 or 70 mg), placebo or OROS-MPH (18, 36 or 54 mg) for a 7-week, double-blind evaluation period. This evaluation period comprised a 4-week, stepwise dose-optimization period (visits 1–4) and a 3-week dose-maintenance period (visits 5–7). It was immediately followed by a 1-week washout and post-treatment safety follow-up (visit 8/FU). Patients who discontinued the study attended an early termination (ET) assessment (data obtained at this visit were included in the visit 7/ET assessment) and a follow-up visit following a 1-week washout period (visit 8/FU). Unused capsules (LDX, placebo or OROS-MPH) were returned and accounted for prior to patients entering the washout period.
Dosing began (at approximately 0700 hours) on the day after completion of the baseline visit (visit 0). Patients initially received LDX 30 mg/day, placebo or OROS-MPH 18 mg/day. If an acceptable response was not achieved, doses were increased in a stepwise manner at weekly intervals at visits 1–3. ‘Acceptable response’ was defined as at least a 30 % reduction in ADHD-RS-IV total score from baseline and a CGI-I score of either 1 (very much improved) or 2 (much improved), with tolerable adverse effects. If a dose was well tolerated and the patient’s symptoms could potentially be improved further, the dose could be increased by one dose level. A reduction of one dose level was permitted if individuals experienced an intolerable adverse effect. Doses could be modified up to but not after visit 3 and patients then continued to take their individually optimized dose for the remainder of the double-blind evaluation period (visits 4–7); patients unable to tolerate the study treatment after this point were withdrawn from the study.
Conners’ Parent Rating Scale-Revised
This study utilized an abbreviated version of the CPRS-R (Multi-Health Systems Inc., North Tonawanda, NY, USA), requiring feedback on 27 symptoms or problem behaviors commonly exhibited by children and adolescents with ADHD, which are grouped into four subscales: ADHD index, oppositional, hyperactivity and cognitive [18]. A parent (or legally appointed representative) gave the symptom or behavior a score of 0–3 as follows: 0, not true at all (never, seldom); 1, just a little true (occasionally); 2, pretty much true (often, quite a bit); 3, very much true (very often, very frequently). Therefore, the total score for the entire scale ranged from 0 to 81. CPRS-R is typically scored based on the patient’s behavior over the past month, but for the purpose of this study, scoring was based on behavior exhibited immediately before the assessment. A decrease in CPRS-R total or subscale score represents an improvement in ADHD symptoms and behaviors.
CPRS-R assessments were initially carried out on the last weekend day before each study visit until a protocol amendment limited assessments to the last weekend day before the baseline visit, visit 4 (day 28) and visit 7 (day 49). To evaluate the duration of therapeutic effect at each study visit and endpoint (defined as the last on-treatment, post-baseline visit with a valid CPRS-R score), CPRS-R assessments were performed at 1000, 1400 and 1800 hours following a single morning dose (administered at approximately 0700 hours). An overall CPRS-R score for each visit was calculated by taking the mean of the three assessments across the day. The present design does not allow for measuring the treatment effect after 1800 hours.
Data analyses
CPRS-R assessments were carried out in the full analysis set (FAS), defined as all patients who were randomized and received at least one dose of investigational product. Patients from one site (n = 15) were excluded owing to violations of good clinical practice. Incomplete data resulting from either early termination or unavailability were handled by the approach of treatment endpoint analysis, in which ‘Endpoint’ is defined as the last on-treatment, post-baseline visit with a valid CPRS-R score.
Statistical analyses included the calculation of least-squares (LS) means and P values, which were based on type III sum of squares from an analysis of covariance (ANCOVA) model for the change from baseline, including treatment, country and age groups as fixed effects and baseline value as covariate. Effect sizes were calculated as the difference between the LS mean scores for the active treatment and placebo groups, divided by the root mean square error obtained from the ANCOVA model. Effect sizes of 0.2, 0.5 and 0.8 are considered to correspond to small, medium and large magnitudes of effect, respectively [19]. OROS-MPH was included in SPD489-325 as a reference arm. The purpose of this was to provide internal validation of the study design and to facilitate interpretation of the data if the study drug (LDX) had failed to show superiority over placebo. However, SPD489-325 was not designed to support a formal statistical comparison between LDX and OROS-MPH.
Results
Patient disposition and baseline demographics
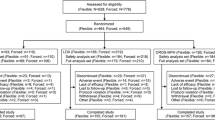
As described previously [15], 336 patients were randomized, 317 were included in the FAS (LDX, n = 104; placebo, n = 106; OROS-MPH, n = 107) and 196 patients completed the study (LDX, n = 80; placebo, n = 42; OROS-MPH, n = 74). In all treatment arms, the most commonly reported reason for discontinuing the study was lack of efficacy (LDX, n = 11; placebo, n = 54; OROS-MPH, n = 22). Baseline demographic and disease characteristics, including mean age (standard deviation [SD]) (LDX, 10.9 [2.9] years; placebo, 11.0 [2.8] years; OROS-MPH, 10.9 [2.6] years) and mean ADHD-RS-IV total score (SD) (LDX, 41.0 [7.3]; placebo, 41.2 [7.2]; OROS-MPH 40.4 [6.8]), were similar among treatment groups [15].
Among patients who had a primary efficacy measurement at endpoint, 26/104 (25.0 %) in the LDX group received the 30-mg dose at endpoint, 38/104 (36.5 %) received 50 mg and 36/104 (34.6 %) received 70 mg. In the OROS-MPH group, 17/107 (15.9 %) patients received the 18-mg dose at endpoint, 22/107 (20.6 %) received 36 mg and 65/107 (60.7 %) received 54 mg.
CPRS-R total score by study visit
Although the protocol was amended during the study to restrict the scheduled CPRS-R assessments to baseline, visit 4 and visit 7, CPRS-R data were available for most of the patients in each group for all visits (Fig. 1a). At baseline, mean (standard error of the mean, SEM) CPRS-R total scores were similar across treatment groups: LDX, 50.9 (1.6); placebo, 53.0 (1.5); OROS-MPH, 51.4 (1.7). In the LDX treatment group, mean CPRS-R total scores decreased during the study (Fig. 1a).The mean (SEM) change from baseline to endpoint in CPRS-R total score was −24.9 (1.8) for LDX and −5.0 (1.3) for placebo. The difference in LS mean change from baseline [95 % confidence interval (CI)] between LDX and placebo was statistically significant at endpoint [P < 0.001, −21.3 (−25.5, −17.0), effect size 1.41] and at each of the on-treatment visits (Fig. 1b). In the OROS-MPH reference arm, the mean (SEM) change from baseline to endpoint in CPRS-R total score was −19.1 (2.1), and the difference in LS mean change from baseline (95 % CI) between OROS-MPH and placebo was statistically significant at endpoint [P < 0.001, −15.1 (−19.3, −10.9), effect size 1.00] and at visits 2–7 (P < 0.05 for visit 6, P ≤ 0.01 for visit 2 and P < 0.001 for visits 3–5 and 7; Fig. 1b).
CPRS-R total score by study visit (full analysis set). a Absolute values and b LS mean changes from baseline. The overall CPRS-R total score for each visit was calculated as the mean of the three assessments across the day. *P < 0.05, **P ≤ 0.01, ***P < 0.001 versus placebo (based on the difference in LS mean change [active treatment − placebo] from baseline to endpoint). Data are presented as mean or LS mean change ± standard error of the mean. Endpoint is the last on-treatment, post-baseline visit with a valid CPRS-R score. Patients enrolled after a protocol amendment had CPRS-R assessments at visits 0, 4 and 7/ET only. A decrease in CPRS-R total score indicates an improvement in ADHD-related symptoms and behaviors. ADHD attention-deficit/hyperactivity disorder, CPRS-R Conners’ Parent Rating Scale-Revised, ET early termination, FU follow-up, LDX lisdexamfetamine dimesylate, LS least-squares, OROS-MPH osmotic-release oral system methylphenidate
CPRS-R by time of day at endpoint
At baseline, mean CPRS-R total scores were within the range 49–54 in all treatment groups and at all assessment times (Fig. 2a). At endpoint, CPRS-R total scores were lower throughout the day in patients treated with LDX than in those treated with placebo (Fig. 2a). Differences in LS mean change from baseline to endpoint (95 % CI) between LDX and placebo were statistically significant (P < 0.001) throughout the day (1000 hours [−21.5 (−25.8, −17.1), effect size 1.42]; 1400 hours [−22.1 (−26.7, −17.6), effect size 1.41]; and 1800 hours [−21.2 (−25.8, −16.5), effect size 1.30], (Fig. 2b). Differences in LS mean change from baseline to endpoint between OROS-MPH and placebo were also statistically significant (P < 0.001) in the morning (−15.6 [−20.0, −11.2], effect size 1.04), afternoon (−15.3 [−19.7, −10.9], effect size 0.98) and evening (−15.0 [−19.7, −10.3], effect size 0.92) (Fig. 2b).
CPRS-R total score at baseline and endpoint by time of day (full analysis set). a Absolute CPRS-R total scores at baseline and endpoint and b LS mean changes from baseline to endpoint in CPRS-R total score by time of day. ***P < 0.001 versus placebo, based on the difference in LS mean change (active treatment − placebo) from baseline to endpoint. Data are presented as mean or LS mean change ± standard error of the mean. Dosing occurred at approximately 0700 hours. Endpoint is the last on-treatment, post-baseline visit with a valid CPRS-R score. Patients enrolled after a protocol amendment had CPRS-R assessments at visits 0, 4 and 7/ET only. A decrease in CPRS-R total score indicates an improvement in ADHD-related symptoms and behaviors. ADHD attention-deficit/hyperactivity disorder, CPRS-R Conners’ Parent Rating Scale-Revised, ET early termination, FU follow-up, LDX lisdexamfetamine dimesylate, OROS-MPH osmotic-release oral system methylphenidate
CPRS-R subscales
Differences in LS mean change from baseline to endpoint between LDX and placebo were statistically significant (P < 0.001) for all four CPRS-R subscale scores (ADHD index, oppositional, hyperactivity and cognitive) averaged across the day (Table 1). Similarly, differences in LS mean from baseline to endpoint between OROS-MPH and placebo were statistically significant (P < 0.001) in all four subscales (Table 1).
Post hoc comparison of LDX and OROS-MPH based on CPRS-R total scores
A statistical comparison between LDX and OROS-MPH was not pre-specified in the SPD489-325 study design. When assessed post hoc, differences in LS mean change from baseline to endpoint (95 % CI) between LDX and OROS-MPH were statistically significant (P < 0.05), in favor of LDX, at 1000 hours (−5.8 [−10.3, −1.4], effect size 0.387), 1400 hours (−6.8 [−11.4, −2.3], effect size 0.435) and 1800 hours (−6.1 [−10.9, −1.4], effect size 0.377).
Discussion
Lisdexamfetamine dimesylate (LDX) was significantly more effective than placebo in reducing symptoms and problem behaviors in children and adolescents with ADHD throughout the 7-week course of this phase 3 study, as assessed using the abbreviated version of the CPRS-R. At endpoint, improvements versus placebo in CPRS-R total score were maintained throughout the day and were ongoing at the last assessment of the day (1800 hours) following a single early morning dose (0700 hours), and benefits were observed across all four CPRS-R subscales (ADHD index, oppositional, hyperactivity and cognitive). Treatment benefits were also observed in the OROS-MPH reference arm, and these were also maintained throughout the day and spanned all four CPRS-R subscales. Stimulant medications, as well as the non-stimulant atomoxetine, are highly effective treatments for ADHD symptoms [20–22]. ADHD symptoms impact the full waking day [23]; hence, the therapeutic duration of action is a primary consideration in ADHD medication choice. Long-acting medications are reported to offer improved convenience, confidentiality, adherence, pharmacokinetic coverage and reduced abuse potential, compared with short-acting counterparts [7, 22, 24, 25].
The prodrug LDX is therapeutically inactive and requires enzymatic cleavage in the blood to yield the active moiety, d-amfetamine [11]. Human pharmacokinetic studies indicate that, after oral administration of LDX, exposure to d-amfetamine is long lasting and dose proportional, with low inter- and intra-individual variability [26, 27]. In the present study, the efficacy of an optimized dose of LDX was evaluated via CPRS-R assessments conducted at three time-points throughout the day. Statistically significant differences between LDX and placebo in LS mean change from baseline to endpoint in CPRS-R total score were observed at all assessment time-points up to and including 1800 hours, following a single early morning dose (0700 hours) of LDX. The CPRS-R total score effect size of 1.30 at the final assessment of the day further suggests that the therapeutic benefit of LDX remains robust for at least 11 h post-dose. However, as the last time-point assessed in the day was at 18.00 h, the design of this study does not allow the duration of effect beyond the 11 h post-dose time-point to be determined.
Results from this first evaluation of LDX in a European sample correspond well with evidence of the long duration of action of LDX derived from North American studies. In the pivotal US study of LDX in children with ADHD, statistically significant differences between LDX and placebo in change from baseline in CPRS-R total score were also ongoing at 1800 hours, following once-daily administration in all three fixed-dose groups (30, 50 and 70 mg) [13]. Further evidence of a long duration of action of LDX comes from a laboratory school study in children with ADHD, in which the efficacy of LDX was maintained for at least 13 h post-dose, as determined by the Swanson, Kotkin, Agler, M-Flynn and Pelham-Attention (SKAMP-A) and -Deportment (SKAMP-D) scales and the Permanent Product Measure of Performance-Attempted (PERMP-A) and -Correct (PERMP-C) scales [16].
The primary efficacy measure of study SPD489-325 was the clinician-rated ADHD-RS-IV, which revealed robust treatment benefits of LDX at endpoint (mean ADHD-RS-IV total score effect size, 1.80). As a parent-rated scale, the CPRS-R complements the ADHD-RS-IV by monitoring ADHD symptoms and problem behaviors outside the clinic, in a range of academic (e.g., homework), social and domestic settings. The value of parent-rated assessments of ADHD symptoms and behaviors is well established [28]. The CPRS was introduced in 1970, and subsequently revised to improve the psychometric properties of the tool [18]. Our observation that patients receiving LDX demonstrated robust improvements across all four subscales of the abbreviated version of the CPRS-R suggests that LDX is effective across a broad range of parent-rated symptom and behavioral domains of ADHD. The large effect sizes obtained in study SPD489-325 for LDX on the CPRS-R (present study) and ADHD-RS-IV [15] are consistent with a previous North American study (range 1.21–1.60) [13] and indicate robust treatment efficacy. It is possible that the relatively small response to placebo in study SPD489-325 also contributed, at least in part, to these large treatment effects.
While the present study did not include an evaluation of subscale scores throughout the day, the US-based, forced-dose, phase 3 clinical trial of LDX in children reported significant improvements in scores of the ADHD index, hyperactivity and cognitive subscales of the CPRS-R at all three assessment times (1000, 1400 and 1800 hours), irrespective of dose [29]. Improvements in the oppositional subscale were significant in all patients treated with LDX at the morning and afternoon assessments only, but were maintained until 1800 hours in the subgroup of patients receiving LDX who had the highest CGI-Severity of Illness scores at baseline (in the range 5–7) [29].
In the present study, OROS-MPH was included as a reference arm. With the exception of the first post-treatment visit (week 1), statistically significant differences versus placebo in LS mean change from baseline in CPRS-R total score were observed in the OROS-MPH treatment group at all visits, with an effect size at endpoint of 1.00. Improvements versus placebo in LS mean change from baseline in CPRS-R total score were evident throughout the day in the OROS-MPH group. Furthermore, improvements were observed across all four CPRS-R subscales. The observed efficacy of OROS-MPH supports the validity and sensitivity of the study design. However, the statistical protocol for this study did not pre-specify a formal statistical comparison between the two active treatments and any comparison of the relative effects of LDX and OROS-MPH must, therefore, be considered exploratory.
Among the key strengths of this study are its randomized, double-blind, placebo-controlled design, the inclusion of an OROS-MPH reference arm, and a European patient population that included both children (aged 6–12 years) and adolescents (aged 13–17 years). The results should, however, be interpreted in the light of several considerations. First, the highly homogeneous study population, from which patients with a range of comorbid diagnoses were excluded, may not reflect a typical cross section of patients with ADHD who are seen in clinical practice. Secondly, while these data indicate that the efficacy of LDX and OROS-MPH is maintained at 1800 hours following a single early morning dose, they do not indicate the maximum therapeutic duration of action of either active treatment, which may have extended beyond this time-point. Finally, although effect sizes relative to placebo were numerically greater for LDX than for OROS-MPH, the study was not powered for inferential statistical comparisons between LDX and OROS-MPH, and because the dose-optimized design of the study precluded any estimation of dose-equivalence, comparisons of the two active treatment arms must be qualitative and tentative.
In conclusion, improvements versus placebo in ADHD-related symptoms and problem behaviors in children and adolescents with ADHD receiving a single early morning dose (0700 hours) of LDX or OROS-MPH were robust throughout the day and were ongoing in the early evening (1800 hours).
References
Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA (2007) The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 164:942–948
Simon V, Czobor P, Balint S, Meszaros A, Bitter I (2009) Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry 194:204–211
Barkley RA, Fischer M, Smallish L, Fletcher K (2002) The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. J Abnorm Psychol 111:279–289
Biederman J, Mick E, Faraone SV (2000) Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am J Psychiatry 157:816–818
World Health Organization (2004) F90 Hyperkinetic disorders. In: ICD-10: International statistical classification of diseases and related health problems, 2nd edn. 10th Revision. World Health Organization, Geneva
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders, 4th edn. Text Revision (DSM-IV-TR). American Psychiatric Association, Washington, DC
Banaschewski T, Coghill D, Santosh P, Zuddas A, Asherson P, Buitelaar J, Danckaerts M, Dopfner M, Faraone SV, Rothenberger A, Sergeant J, Steinhausen HC, Sonuga-Barke EJ, Taylor E (2006) Long-acting medications for the hyperkinetic disorders. A systematic review and European treatment guideline. Eur Child Adolesc Psychiatry 15:476–495
National Institute for Health and Clinical Excellence (2009) Diagnosis and management of ADHD in children, young people and adults. National Clinical Practice Guideline Number 72, London, UK. http://wwwniceorguk/nicemedia/live/12061/42060/42060pdf
Wolraich M, Brown L, Brown RT, DuPaul G, Earls M, Feldman HM, Ganiats TG, Kaplanek B, Meyer B, Perrin J, Pierce K, Reiff M, Stein MT, Visser S (2011) ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 128:1007–1022
Taylor E, Dopfner M, Sergeant J, Asherson P, Banaschewski T, Buitelaar J, Coghill D, Danckaerts M, Rothenberger A, Sonuga-Barke E, Steinhausen HC, Zuddas A (2004) European clinical guidelines for hyperkinetic disorder—first upgrade. Eur Child Adolesc Psychiatry 13(Suppl 1):I7–I30
Pennick M (2010) Absorption of lisdexamfetamine dimesylate and its enzymatic conversion to d-amphetamine. Neuropsychiatr Dis Treat 6:317–327
Adler LA, Goodman DW, Kollins SH, Weisler RH, Krishnan S, Zhang Y, Biederman J (2008) Double-blind, placebo-controlled study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry 69:1364–1373
Biederman J, Krishnan S, Zhang Y, McGough JJ, Findling RL (2007) Efficacy and tolerability of lisdexamfetamine dimesylate (NRP-104) in children with attention-deficit/hyperactivity disorder: a phase III, multicenter, randomized, double-blind, forced-dose, parallel-group study. Clin Ther 29:450–463
Findling RL, Childress AC, Cutler AJ, Gasior M, Hamdani M, Ferreira-Cornwell MC, Squires L (2011) Efficacy and safety of lisdexamfetamine dimesylate in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 50:395–405
Coghill DR, Banaschewski T, Lecendreux M, Soutullo C, Johnson M, Zuddas A, Anderson CA, Civil R, Higgins CN, Lyne A, Squires L (2013) European, randomized, phase 3 study of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol. doi:10.1016/j.euroneuro.2012.11.012
Wigal SB, Kollins SH, Childress AC, Squires L (2009) A 13-hour laboratory school study of lisdexamfetamine dimesylate in school-aged children with attention-deficit/hyperactivity disorder. Child Adolesc Psychiatry Ment Health 3:17
Wigal T, Brams M, Gasior M, Gao J, Squires L, Giblin J (2010) Randomized, double-blind, placebo-controlled, crossover study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder: novel findings using a simulated adult workplace environment design. Behav Brain Funct 6:34
Conners CK, Sitarenios G, Parker JD, Epstein JN (1998) The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol 26:257–268
Cohen J (1992) A power primer. Psychol Bull 112:155–159
Elia J (2005) Attention deficit/hyperactivity disorder: pharmacotherapy. Psychiatry (Edgmont) 2:27–35
Garnock-Jones KP, Keating GM (2009) Atomoxetine: a review of its use in attention-deficit hyperactivity disorder in children and adolescents. Paediatr Drugs 11:203–226
May DE, Kratochvil CJ (2010) Attention-deficit hyperactivity disorder: recent advances in paediatric pharmacotherapy. Drugs 70:15–40
Coghill D, Soutullo C, d’Aubuisson C, Preuss U, Lindback T, Silverberg M, Buitelaar J (2008) Impact of attention-deficit/hyperactivity disorder on the patient and family: results from a European survey. Child Adolesc Psychiatry Ment Health 2:31
Findling RL (2008) Evolution of the treatment of attention-deficit/hyperactivity disorder in children: a review. Clin Ther 30:942–957
Pliszka S (2007) Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 46:894–921
Boellner SW, Stark JG, Krishnan S, Zhang Y (2010) Pharmacokinetics of lisdexamfetamine dimesylate and its active metabolite, d-amphetamine, with increasing oral doses of lisdexamfetamine dimesylate in children with attention-deficit/hyperactivity disorder: a single-dose, randomized, open-label, crossover study. Clin Ther 32:252–264
Ermer J, Homolka R, Martin P, Buckwalter M, Purkayastha J, Roesch B (2010) Lisdexamfetamine dimesylate: linear dose-proportionality, low intersubject and intrasubject variability, and safety in an open-label single-dose pharmacokinetic study in healthy adult volunteers. J Clin Pharmacol 50:1001–1010
Biederman J, Faraone SV, Monuteaux MC, Grossbard JR (2004) How informative are parent reports of attention-deficit/hyperactivity disorder symptoms for assessing outcome in clinical trials of long-acting treatments? A pooled analysis of parents’ and teachers’ reports. Pediatrics 113:1667–1671
Lopez FA, Ginsberg LD, Arnold V (2008) Effect of lisdexamfetamine dimesylate on parent-rated measures in children aged 6 to 12 years with attention-deficit/hyperactivity disorder: a secondary analysis. Postgrad Med 120:89–102
Acknowledgments
We thank the patients and investigators who took part in this study. We thank Dr. Eric Southam of Oxford PharmaGenesis™ Ltd. for editorial assistance, collating the comments of authors and editing the paper for submission. This study was supported by funding from Shire Development LLC.
Conflict of interest
This study was supported by funding from Shire Development LLC. R Civil, R Bloomfield and LA Squires are employees of Shire. The following authors have received compensation for serving as consultants or speakers, or they or the institutions they work for have received research support or royalties from the companies or organizations indicated: DR Coghill (Flynn, Janssen-Cilag, Lilly, Medice, Novartis, Otsuka, Oxford University Press, Pfizer, Schering-Plough, Shire, UCB, Vifor Pharma); T Banaschewski (Bristol-Myers Squibb, Desitin, Janssen McNeil, Lilly, Medice, Novartis, Pfizer, Shire, UCB, Vifor Pharma); M Lecendreux (Shire, UCB, Vifor Pharma); A Zuddas (EU/Th Framework, Italian National Institute of Health, Sardinian Secretary of Health, AstraZeneca, Bristol-Myers Squibb/Otsuka, Lilly, Lundbeck, Shire, Vifor Pharma); RW Dittmann (EU, US National Institute of Mental Health, German Federal Ministry of Health/Regulatory Agency, German Federal Ministry of Education and Research, German Research Foundation, Ferring, Janssen-Cilag, Lilly, Otsuka, Shire); I Hernández Otero (Janssen-Cilag, Junta de Andalucia, Lilly, Roche, Shire).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Coghill, D.R., Banaschewski, T., Lecendreux, M. et al. Efficacy of lisdexamfetamine dimesylate throughout the day in children and adolescents with attention-deficit/hyperactivity disorder: results from a randomized, controlled trial. Eur Child Adolesc Psychiatry 23, 61–68 (2014). https://doi.org/10.1007/s00787-013-0421-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00787-013-0421-y