Abstract
Objective
This study aimed to evaluate the Wnt/β-catenin signaling pathway activity in gingival samples obtained from patients with periodontitis.
Materials and methods
Fifteen patients with stage III grade B (SIIIGB) and eleven with stage III grade C (SIIIGC) periodontitis were included and compared to 15 control subjects. β-Catenin, Wnt 3a, Wnt 5a, and Wnt 10b expressions were evaluated by Q-PCR. Topographic localization of tissue β-catenin, Wnt 5a, and Wnt 10b was measured by immunohistochemical analysis. TNF-α was used to assess the inflammatory state of the tissues, while Runx2 was used as a mediator of active destruction.
Results
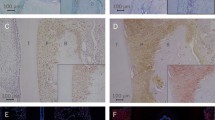
Wnt 3a, Wnt 5a, and Wnt 10b were significantly higher in gingival tissues in both grades of stage 3 periodontitis compared to the control group (p < 0.05). β-Catenin showed intranuclear staining in connective tissue in periodontitis, while it was confined to intracytoplasmic staining in epithelial tissue and the cell walls in the control group. Wnt5a protein expression was elevated in periodontitis, with the most intense staining observed in the connective tissue of SIIIGC samples. Wnt10b showed the highest density in the connective tissue of patients with periodontitis.
Conclusions
Our findings suggested that periodontal inflammation disrupts the Wnt/β-catenin signaling pathway.
Clinical Relevance
Periodontitis disrupts Wnt signaling in periodontal tissues in parallel with tissue inflammation and changes in morphology. This change in Wnt-related signaling pathways that regulate tissue homeostasis in the immunoinflammatory response may shed light on host-induced tissue destruction in the pathogenesis of the periodontal disease.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Notes
RNAlater® Tissue Collection: RNA Stabilization Solution, Applied Biosystems, California, USA.
Cat# ab174963, Abcam, Cambridge, UK.
Cat# ab189030, Abcam, Cambridge, UK.
Cat#ab224803, Abcam, Cambridge, UK.
Ventana Medical Systems, Arizona, USA.
Roche Diagnostics, Basel, Switzerland.
TriPure® Isolation Reagent, Roche, Mannheim, Germany.
NanoDrop™ One/OneC Microvolume UV–Vis Spectrophotometer, Thermo Fisher Scientific, Wilmington, USA.
Roche, Mannheim, Germany.
Roche Diagnostics, Basel, Switzerland.
Roche Diagnostics, Basel, Switzerland.
3.1.9.2 G*Power; https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und arbeitspsychologie/gpower.html.
SPSS for Windows v.26, IBM SPSS Inc., New York, NY, USA.
References
Bonewald LF, Johnson ML (2008) Osteocytes, mechanosensing and Wnt signaling. Bone 42:606–615. https://doi.org/10.1016/j.bone.2007.12.224
Nagafuchi A, Takeichi M (1989) Transmembrane control of cadherin-mediated cell adhesion: a 94 kDa protein functionally associated with a specific region of the cytoplasmic domain of E-cadherin. Cell Regul 1:37–44. https://doi.org/10.1091/mbc.1.1.37
McCrea PD, Turck CW, Gumbiner B (1991) A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science 254:1359–1361. https://doi.org/10.1126/science.1962194
Kemler R (1993) From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet 9:317–321. https://doi.org/10.1016/0168-9525(93)90250-l
Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, Kemler R (1996) Nuclear localization of beta-catenin by interaction with transcription factor LEF-1. Mech Dev 59:3–10. https://doi.org/10.1016/0925-4773(96)00597-7
Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destrée O, Clevers H (1996) XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell 86:391–399. https://doi.org/10.1016/s0092-8674(00)80112-9
Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA 2nd, Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L (2002) Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol 157:303–314. https://doi.org/10.1083/jcb.200201089
Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Jüppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML, Osteoporosis-Pseudoglioma Syndrome Collaborative Group (2001) LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107:513–23. https://doi.org/10.1016/s0092-8674(01)00571-2
Westendorf JJ, Kahler RA, Schroeder TM (2004) Wnt signaling in osteoblasts and bone diseases. Gene 27:19–39. https://doi.org/10.1016/j.gene.2004.06.044
Darveau RP, Curtis MA (2021) Oral biofilms revisited: a novel host tissue of bacteriological origin. Periodontol 2000 86:8–13. https://doi.org/10.1111/prd.12374
Joseph S, Curtis MA (2021) Microbial transitions from health to disease. Periodontol 2000 86:201–9. https://doi.org/10.1111/prd.12377
Tang Y, Zhou X, Gao B, Xu X, Sun J, Cheng L, Zhou X, Zheng L (2014) Modulation of Wnt/β-catenin signaling attenuates periapical bone lesions. J Dent Res 93:175–182. https://doi.org/10.1177/0022034513512507
Pereira C, Schaer DJ, Bachli EB, Kurrer MO, Schoedon G (2008) Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the anti-inflammatory action of activated protein C and interleukin-10. Arterioscler Thromb Vasc Biol 28:504–510. https://doi.org/10.1161/ATVBAHA.107.157438
Chatzopoulos GS, Costalonga M, Mansky KC, Wolff LF (2021) WNT-5a and SOST levels in gingival crevicular fluid depend on the inflammatory and osteoclastogenic activities of periodontal tissues. Medicina (Kaunas) 31(57):788. https://doi.org/10.3390/medicina57080788
Chatzopoulos GS, Mansky KC, Lunos S, Costalonga M, Wolff LF (2019) Sclerostin and WNT-5a gingival protein levels in chronic periodontitis and health. J Periodontal Res 54:555–565. https://doi.org/10.1111/jre.12659
Baksh D, Boland GM, Tuan RS (2007) Cross-talk between Wnt signaling pathways in human mesenchymal stem cells leads to functional antagonism during osteogenic differentiation. J Cell Biochem 101:1109–1124. https://doi.org/10.1002/jcb.21097
Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ (1997) Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89:765–771. https://doi.org/10.1016/s0092-8674(00)80259-7
Ogawa E, Maruyama M, Kagoshima H, Inuzuka M, Lu J, Satake M, Shigesada K, Ito Y (1993) PEBP2/PEA2 represents a family of transcription factors homologous to the products of the Drosophila runt gene and the human AML1 gene. Proc Natl Acad Sci U S A 90:6859–6863. https://doi.org/10.1073/pnas.90.14.6859
Liu X, Zhang Z, Pan S, Shang S, Li C (2018) Interaction between the Wnt/β-catenin signaling pathway and the EMMPRIN/MMP-2, 9 route in periodontitis. J Periodontal Res 53:842–852. https://doi.org/10.1111/jre.12574
Iizumi R, Honda M (2022) Wnt/β-catenin signaling ınhibits osteogenic differentiation in human periodontal ligament fibroblasts. Biomimetics (Basel) 7:224. https://doi.org/10.3390/biomimetics7040224
Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, MacDougald OA (2005) Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A 102:3324–3329. https://doi.org/10.1073/pnas.0408742102
Lima MDR, Lopes AP, Martins C, Brito GAC, Carneiro VC, Goes P (2017) The effect of Calendula officinalis on oxidative stress and bone loss in experimental periodontitis. Front Physiol 8:440. https://doi.org/10.3389/fphys.2017.00440
Kebschull M, Demmer RT, Grün B, Guarnieri P, Pavlidis P, Papapanou PN (2014) Gingival tissue transcriptomes identify distinct periodontitis phenotypes. J Dent Res 93:459–468. https://doi.org/10.1177/0022034514527288
Tonetti MS, Greenwell H, Kornman KS (2018) Staging and grading of periodontitis: framework and proposal of a new classification and case definition. Review J Periodontol 89:159–172. https://doi.org/10.1111/jcpe.12945
Armitage GC (1999) Development of a classification system for periodontal diseases and conditions. Ann Periodontol 4:1–6. https://doi.org/10.1902/annals.1999.4.1.1
Onder C, Kurgan S, Balci N, Guney Z, Serdar MA, Gunhan O, Gunhan M (2016) Vascular endothelial growth factor levels (VEGF) in gingival biopsies of patients with chronic or aggressive periodontitis. J Ponte 72. https://doi.org/10.21506/j.ponte.2016.7.19
Topol L, Chen W, Song H, Day TF, Yang Y (2009) Sox9 inhibits wnt signaling by promoting β-catenin phosphorylation in the nucleus. J Biol Chem 284:3323–3333. https://doi.org/10.1074/jbc.M808048200
Slusarski DC, Yang-Snyder J, Busa WB, Moon RT (1997) Modulation of embryonic intracellular Ca2+signaling byWnt-5A. Dev Biol 182:114–120. https://doi.org/10.1006/dbio.1996.8463
Sheldahl LC, Park M, Malbon CC, Moon RT (1999) Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Curr Biol 9:695–698. https://doi.org/10.1016/s0960-9822(99)80310-8
Tervahartiala T, Koski H, Xu JW, Häyrinen-Immonen R, Hietanen J, Sorsa T, Konttinen YT (2001) Tumor necrosis factor-alpha and its receptors, p55 and p75, in gingiva of adult periodontitis. J Dent Res 80:1535–1539. https://doi.org/10.1177/00220345010800061101
de Oliveira RR, Schwartz-Filho HO, Novaes AB, Garlet GP, de Souza RF, Taba M, Scombatti de Souza SL, Ribeiro FJ (2009) Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: cytokine profile in gingival crevicular fluid, preliminary results. J Periodontol 80:98–105. https://doi.org/10.1902/jop.2009.070465
Papadopoulos G, Weinberg EO, Massari P, Gibson FC 3rd, Wetzler LM, Morgan EF, Genco CA (2013) Macrophage-specific TLR2 signaling mediates pathogen-induced TNF-dependent inflammatory oral bone loss. J Immunol 190:1148–1157. https://doi.org/10.4049/jimmunol.1202511
Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, Javed A, van Wijnen AJ, Stein JL, Stein GS, Lian JB (2005) Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem 280:33132–33140. https://doi.org/10.1074/jbc.M500608200
Afacan B, Ilhan HA, Köse T, Emingil G (2023) Gingival crevicular fluid galectin-3 and interleukin-1 beta levels in stage 3 periodontitis with grade B and C. Clin Oral Investig. https://doi.org/10.1007/s00784-023-04991-7
Goh KI, Cusick ME, Valle D, Childs B, Vidal M, Barabási AL (2007) The human disease network. Proc Natl Acad Sci U S A 104:8685–8690. https://doi.org/10.1073/pnas.0701361104
Lage K, Hansen NT, Karlberg EO, Eklund AC, Roque FS, Donahoe PK, Szallasi Z, Jensen TS, Brunak S (2008) A large-scale analysis of tissue-specific pathology and gene expression of human disease genes and complexes. Proc Natl Acad Sci U S A 105:20870–20875. https://doi.org/10.1073/pnas.0810772105
Pierson E, GTEx Consortium, Koller D, Battle A, Mostafavi S, Ardlie KG, Getz G, Wright FA, Kellis M, Volpi S, Dermitzakis ET (2015) Sharing and specificity of co-expression networks across 35 human tissues. PLoS Comput Biol 11(5):e1004220. https://doi.org/10.1371/journal.pcbi.1004220
Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, Powell JI, Yang L, Marti GE, Moore T, Hudson J Jr, Lu L, Lewis DB, Tibshirani R, Sherlock G, Chan WC, Greiner TC, Weisenburger DD, Armitage JO, Warnke R, Levy R, Wilson W, Grever MR, Byrd JC, Botstein D, Brown PO, Staudt LM (2000) Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403:503–511. https://doi.org/10.1038/35000501
Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lønning PE, Børresen-Dale AL, Brown PO, Botstein D (2000) Molecular portraits of human breast tumours. Nature 406:747–752. https://doi.org/10.1038/35021093
Haslett JN, Kunkel LM (2002) Microarray analysis of normal and dystrophic skeletal muscle. Int J Dev Neurosci 20:359–365. https://doi.org/10.1016/s0736-5748(02)00041-2
Colangelo V, Schurr J, Ball MJ, Pelaez RP, Bazan NG, Lukiw WJ (2002) Gene expression profiling of 12633 genes in Alzheimer hippocampal CA1: transcription and neurotrophic factor down-regulation and up-regulation of apoptotic and pro-inflammatory signaling. J Neurosci Res 70:462–473. https://doi.org/10.1002/jnr.10351
Kebschull M, Guarnieri P, Demmer RT, Boulesteix AL, Pavlidis P, Papapanou PN (2013) Molecular differences between chronic and aggressive periodontitis. J Dent Res 92:1081–1088. https://doi.org/10.1177/0022034513506011
Willert K, Jones KA (2006) Wnt signaling: is the party in the nucleus? Genes Dev 20:1394–1404. https://doi.org/10.1101/gad.1424006
Zhu L, Yao Y, Liu J, Wang J, Xie H (2019) Expression of β-catenin and MMP-8 in gingival crevicular fluid and gingival tissue indicates the disease severity of patients with chronic periodontitis. Exp Ther Med 18:2131–2139. https://doi.org/10.3892/etm.2019.7794
Nakashima A, Tamura M (2006) Regulation of matrix metalloproteinase-13 and tissue inhibitor of matrix metalloproteinase-1 gene expression by WNT3A and bone morphogenetic protein-2 in osteoblastic differentiation. Front Biosci 11:1667–1678. https://doi.org/10.2741/1912
Armitage GC (2000) Development of a classification system for periodontal diseases and conditions. Northwest Dent 79:31–55
Fine DH, Markowitz K, Fairlie K, Tischio-Bereski D, Ferrandiz J, Godboley D, Furgang D, Gunsolley J, Best A (2014) Macrophage inflammatory protein-1α shows predictive value as a risk marker for subjects and sites vulnerable to bone loss in a longitudinal model of aggressive periodontitis. PLoS One 9:e98541. https://doi.org/10.1371/journal.pone.0098541
Nanbara H, Wara-aswapati N, Nagasawa T, Yoshida Y, Yashiro R, Bando Y, Kobayashi H, Khongcharoensuk J, Hormdee D, Pitiphat W, Boch JA, Izumi Y (2012) Modulation of Wnt5a expression by periodontopathic bacteria. PLoS One 7:e34434. https://doi.org/10.1371/journal.pone.0034434
Divaris K, Monda KL, North KE, Olshan AF, Reynolds LM, Hsueh WC, Lange EM, Moss K, Barros SP, Weyant RJ, Liu Y, Newman AB, Beck JD, Offenbacher S (2013) Exploring the genetic basis of chronic periodontitis: a genome-wide association study. Hum Mol Genet 22:2312–2324. https://doi.org/10.1093/hmg/ddt065
Maekawa T, Kulwattanaporn P, Hosur K, Domon H, Oda M, Terao Y, Maeda T, Hajishengallis G (2017) Differential expression and roles of secreted frizzled-related protein 5 and the wingless homolog wnt5a in periodontitis. J Dent Res 96:571–577. https://doi.org/10.1177/0022034516687248
Blumenthal A, Ehlers S, Lauber J, Buer J, Lange C, Goldmann T, Heine H, Brandt E, Reiling N (2006) The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood 108:965–973. https://doi.org/10.1182/blood-2005-12-5046
Maeda K, Kobayashi Y, Udagawa N, Uehara S, Ishihara A, Mizoguchi T, Kikuchi Y, Takada I, Kato S, Kani S, Nishita M, Marumo K, Martin TJ, Minami Y, Takahashi N (2012) Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat Med 18:405–412. https://doi.org/10.1038/nm.2653
Zhao Y, Zhang C, Huang Y, Yu Y, Li R, Li M, Liu N, Liu P, Qiao J (2015) Up-regulated expression of WNT5a increases inflammation and oxidative stress via PI3K/AKT/NF-κB signaling in the granulosa cells of PCOS patients. J Clin Endocrinol Metab 100:201–211. https://doi.org/10.1210/jc.2014-2419
Zhao H, Huang Y, Tao J (2016) ST1926 Attenuates steroid-induced osteoporosis in rats by inhibiting inflammation response. J Cell Biochem 118:2072–2086. https://doi.org/10.1002/jcb.25812
Artese L, Simon MJ, Piattelli A, Ferrari DS, Cardoso LA, Faveri M, Onuma T, Piccirilli M, Perrotti V, Shibli JA (2011) Immunohistochemical analysis of inflammatory infiltrate in aggressive and chronic periodontitis: a comparative study. Clin Oral Investig 15:233–240. https://doi.org/10.1007/s00784-009-0374-1
Halleskog C, Dijksterhuis JP, Kilander MB, Becerril-Ortega J, Villaescusa JC, Lindgren E, Arenas E, Schulte G (2012) Heterotrimeric G protein-dependent WNT-5A signaling to ERK1/2 mediates distinct aspects of microglia proinflammatory transformation. J Neuroinflammation 9:111. https://doi.org/10.1186/1742-2094-9-111
Halleskog C, Mulder J, Dahlström J, Mackie K, Hortobágyi T, Tanila H, Kumar Puli L, Färber K, Harkany T, Schulte G (2011) WNT signaling in activated microglia is proinflammatory. Glia 59:119–131. https://doi.org/10.1002/glia.21081
Halleskog C, Schulte G (2013) Pertussis toxin-sensitive heterotrimeric G(ai/o) proteins mediate WNT/b-catenin and WNT/ERK1/2 signaling in mouse primary microglia stimulated with purified WNT-3A. Cell Signal 25:822–828. https://doi.org/10.1016/j.cellsig.2012.12.006
Chen Y, Alman BA (2009) Wnt pathway, an essential role in bone regeneration. J Cell Biochem 106:353–362. https://doi.org/10.1002/jcb.22020
Lima MDR, Lopes AP, Martins C, Brito GAC, Carneiro VC, Goes P (2017) The effect of calendula officinalis on oxidative stress and bone loss in experimental periodontitis. Front Physiol 28:440. https://doi.org/10.3389/fphys.2017.00440
Nemoto E, Koshikawa Y, Kanaya S, Tsuchiya M, Tamura M, Somerman MJ, Shimauchi H (2009) Wnt signaling inhibits cementoblast differentiation and promotes proliferation. Bone 44:805–812. https://doi.org/10.1016/j.bone.2008.12.029
Geoffroy V, Kneissel M, Fournier B, Boyde A, Matthias P (2002) High bone resorption in adult aging transgenic mice overexpressing cbfa1/runx2 in cells of the osteoblastic lineage. Mol Cell Biol 22:6222–6233. https://doi.org/10.1128/MCB.22.17.6222-6233.2002
Li K, Dong SG, Zhang HX, Zhou S, Ma L, Yu QQ, Jiang ZY, Hu QF, Zhou D (2016) Expression of RUNX2 and MDM21 in rats with periodontitis under chronic intermittent hypoxia. Asian Pac J Trop Med 9:781–785. https://doi.org/10.1016/j.apjtm.2016.06.002
Bleil J, Maier R, Hempfing A, Sieper J, Appel H, Syrbe U (2016) Granulation tissue eroding the subchondral bone also promotes new bone formation in ankylosing spondylitis. Arthritis Rheumatol 68(10):2456–2465. https://doi.org/10.1002/art.39715
Arabaci T, Cicek Y, Canakci V, Canakci CF, Ozgoz M, Albayrak M, Keles ON (2010) Immunohistochemical and stereologic analysis of NF-κB activation in chronic periodontitis. Eur J Dent 4:454–461
Hajishengallis G (2014) Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol 35:3–11. https://doi.org/10.1016/j.it.2013.09.001
Acknowledgements
The authors would like to thank Assoc. Prof. Armağan Günal for his help in the IHC analyses.
Funding
This research was supported by project 17H0234001 of the Ankara University Scientific Research Projects Office.
Author information
Authors and Affiliations
Contributions
ZG contributed to the study design, collected samples, recorded clinical data, helped interpret the results, and wrote the manuscript with input from other authors. SK contributed to the study design, helped to collect tissue samples, helped interpret the results, and wrote the manuscript with input from other authors. CO contributed to the study design, helped interpret the results, and wrote the manuscript with input from other authors. MAT helped with samples collection. ÖG contributed to immunohistochemical analysis and study design and helped interpret the results. MAS contributed to biochemical analysis and study design performed the statistical analysis and helped interpret the results. AK contributed to directing the implementation of the research and assisted with the interpretation of results and manuscript revision. MG contributed to study design, overseeing the implementation of the study, and helped with the interpretation of results and manuscript revision. All authors reviewed and approved the submitted final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The design and the consent for this study were approved by the Ethics Committee on Human Research of Ankara University (no: 36290600/52, on 26.05.2017).
Informed consent
All individuals provided written informed consent.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Güney, Z., Kurgan, Ş., Önder, C. et al. Wnt signaling in periodontitis. Clin Oral Invest 27, 6801–6812 (2023). https://doi.org/10.1007/s00784-023-05294-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-023-05294-7