Abstract
Objectives
Aging is characterized by chronic inflammatory activity. Senescent cells increase with chronic inflammation and age-related pathologies, including periodontal disease. As a critical regulator of tissue inflammaging, we hypothesized that 5α reductase (5αR) is associated with periodontal disease and bacteria-induced senescence in gingival fibroblasts.
Materials and methods
We recruited 36 patients with periodontitis, measured 5αR immunohistochemically before and after periodontal treatment, and compared the expression of 5αR in gingival biopsies from 12 healthy individuals. We then tested the impact of Porphyromonas gingivalis on gingival fibroblasts treated with or without D-galactose-induced cell senescence. We treated primary gingival fibroblasts with D-galactose-supplemented media (0 µM, 50 µM, 100 µM, 1 mM, 10 mM, 50 mM) to induce senescence. The expression of type 1 and type 2 5αR was analyzed with real-time PCR and immunocytochemistry. The levels of IL-6, IL-8, TNF-α, and MCP-1 in fibroblast cultures were evaluated by multiplex immunoassay.
Results
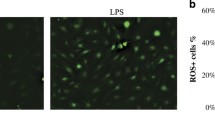
In gingival biopsies from patients with periodontal disease, the expression of 5αR was significantly higher than in samples from individuals without periodontal disease (p < 0.001). Periodontal treatment significantly reduced the expression of 5αR in gingival tissues (p < 0.001) to levels comparable in healthy individuals. Gingival fibroblasts exposed to D-galactose-supplemented media had a dose-dependent and significant increase in 5αR expression (p < 0.001). P. gingivalis caused statistically higher type 1 and type 2 5αR expression in gingival fibroblast cells. This effect was exacerbated by the lower doses of D-galactose (p = 0.037). Cells infected with P. gingivalis produced significantly higher levels of IL-6, IL-8, TNF-α, and MCP-1 (p < 0.05) regardless of the D-galactose exposure.
Conclusion
The results suggested that 5αR plays a role in periodontal disease and mediates the senescence-induced response to P. gingivalis in gingival fibroblasts.
Clinical relevance
Periodontal diseases and aging can increase the production of 5-alpha reductase in the gingival tissue.





Similar content being viewed by others
Data availability
The data which obtain from patient supporting this study cannot been made available due to ethical and legal.
References
Mariotti A (1994) Sex steroid hormones and cell dynamics in the periodontium. Crit Rev Oral Biol Med 5(1):27–53. https://doi.org/10.1177/10454411940050010201
Dohle GR, Smit M, Weber RF (2003) Androgens and male fertility. World J Urol 21(5):341–345. https://doi.org/10.1007/s00345-003-0365-9
Zouboulis CC (2004) The human skin as a hormone target and an endocrine gland. Hormones (Athens) 3(1):9–26. https://doi.org/10.14310/horm.2002.11109
Kasperk CH, Wergedal JE, Farley J, Linkhart TA, Turner RT, Baylink DJ (1989) Androgens directly stimulate proliferation of bone cells in vitro. Endocrinology 124(3):1576–1578. https://doi.org/10.1210/endo-124-3-1576
Sultan C, Oliel V, Audran F, Meynadier J (1986) Free and total plasma testosterone in men and women with acne. Acta Derm Venereol 66(4):301–304
Asada Y, Sonoda T, Ojiro M, Kurata S, Sato T, Ezaki T, Takayasu S (2001) 5 alpha-reductase type 2 is constitutively expressed in the dermal papilla and connective tissue sheath of the hair follicle in vivo but not during culture in vitro. J Clin Endocrinol Metab 86(6):2875–2880. https://doi.org/10.1210/jcem.86.6.7545
Springer K, Brown M, Stulberg DL (2003) Common hair loss disorders. Am Fam Physician 68(1):93–102
Tindall DJ, Rittmaster RS (2008) The rationale for inhibiting 5alpha-reductase isoenzymes in the prevention and treatment of prostate cancer. J Urol 179(4):1235–1242. https://doi.org/10.1016/j.juro.2007.11.033
Gavazzi G, Krause KH (2002) Ageing and infection. Lancet Infect Dis 2(11):659–666. https://doi.org/10.1016/s1473-3099(02)00437-1
Diamanti-Kandarakis E, Dattilo M, Macut D, Duntas L, Gonos ES, Goulis DG et al (2017) Mechanisms in endocrinology: aging and anti-aging: a combo-endocrinology overview. Eur J Endocrinol 176(6):283–308. https://doi.org/10.1530/EJE-16-1061
Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G (2000) Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 908:244–254. https://doi.org/10.1111/j.1749-6632.2000.tb06651.x
Coppé JP, Desprez PY, Krtolica A, Campisi J (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5:99–118. https://doi.org/10.1146/annurev-pathol-121808-102144
Noren Hooten N, Evans MK (2017) Techniques to induce and quantify cellular senescence. J Vis Exp 123:55533. https://doi.org/10.3791/55533
Salama R, Sadaie M, Hoare M, Narita M (2014) Cellular senescence and its effector programs. Genes Dev 28(2):99–114. https://doi.org/10.1101/gad.235184.113
Dodig S, Čepelak I, Pavić I (2019) Hallmarks of senescence and aging. Biochem Med (Zagreb) 29(3):030501. https://doi.org/10.11613/BM.2019.030501
Lasry A, Ben-Neriah Y (2015) Senescence-associated inflammatory responses: aging and cancer perspectives. Trends Immunol 36(4):217–228. https://doi.org/10.1016/j.it.2015.02.009
Shelton DN, Chang E, Whittier PS, Choi D, Funk WD (1999) Microarray analysis of replicative senescence. Curr Biol 9(17):939–945. https://doi.org/10.1016/s0960-9822(99)80420-5
Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Ross OA (2018) Age and age-related diseases: role of inflammation triggers and cytokines. Front Immunol 9:586. https://doi.org/10.3389/fimmu.2018.00586
Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salviol S (2007) Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 128(1):92–105. https://doi.org/10.1016/j.mad.2006.11.016
Abiko Y, Shimizu N, Yamaguchi M, Suzuki H, Takiguchi H (1998) Effect of aging on functional changes of periodontal tissue cells. Ann Periodontol 3(1):350–369. https://doi.org/10.1902/annals.1998.3.1.350
Mascarenhas P, Gapski R, Al-Shammari K, Wang HL (2003) Influence of sex hormones on the periodontium. J Clin Periodontol 30(8):671–681. https://doi.org/10.1034/j.1600-051x.2003.00055.x
Markou E, Eleana B, Lazaros T, Antonios K (2009) The influence of sex steroid hormones on gingiva of women. Open Dent J 3:114–119. https://doi.org/10.2174/1874210600903010114
Sooriyamoorthy M, Gower DB (1989) Hormonal influences on gingival tissue: relationship to periodontal disease. J Clin Periodontol 16(4):201–208. https://doi.org/10.1111/j.1600-051x.1989.tb01642.x
Steffens JP, Wang X, Starr JR, Spolidorio LC, Van Dyke TE, Kantarci A (2015) Associations between sex hormone levels and periodontitis in men: results from NHANES III. J Periodontol 86(10):1116–1125. https://doi.org/10.1902/jop.2015.140530
Steffens JP, Herrera BS, Coimbra LS, Stephens DN, Rossa C Jr, Spolidorio LC, Kantarci A, Van Dyke TE (2014) Testosterone regulates bone response to inflammation. Horm Metab Res 46(3):193–200. https://doi.org/10.1055/s-0034-1367031
Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH (2004) The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab 89(7):3313–3318. https://doi.org/10.1210/jc.2003-031069
Parkar M, Tabona P, Newman H, Olsen I (1998) IL-6 expression by oral fibroblasts is regulated by androgen. Cytokine 10(8):613–619. https://doi.org/10.1006/cyto.1998.0336
Kasasa SC, Soory M (1995) The response of human gingival fibroblasts to interleukin-1 in their metabolic conversion of two androgenic substrates. Arch Oral Biol 40(10):979–981. https://doi.org/10.1016/0003-9969(95)00058-w
Clark DT, Soory M (2007) Steroid 5alpha-reductase activity of Treponema denticola. Oral Microbiol Immunol 22(5):326–332. https://doi.org/10.1111/j.1399-302X.2007.00364.x
Soory M, Ahmad S (1997) 5 alpha reductase activity in human gingiva and gingival fibroblasts in response to bacterial culture supernatants, using [14C]4-androstenedione as substrate. Arch Oral Biol 42(4):255–262. https://doi.org/10.1016/s0003-9969(97)00028-9
Sato Y, Atarashi K, Plichta DR, Arai Y, Sasajima S, Kearney SM, Suda W et al (2021) Novel bile acid biosynthetic pathways are enriched in the microbiome of centenarians. Nature 599(7885):458–464. https://doi.org/10.1038/s41586-021-03832-5
Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH et al (2018) Periodontitis: consensus report of workgroup 2 of the 2017 World Workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol 89(Suppl 1):173–182. https://doi.org/10.1002/JPER.17-0721
Bozkurt SB, Hakki SS, Hakki EE, Durak Y, Kantarci A (2017) Porphyromonas gingivalis lipopolysaccharide induces a pro-inflammatory human gingival fibroblast phenotype. Inflammation 40(1):144–153. https://doi.org/10.1007/s10753-016-0463-7
Lolli F, Pallotti F, Rossi A, Fortuna MC, Caro G, Lenzi A, Sansone A, Lombardo F (2017) Androgenetic alopecia: a review. Endocrine 57(1):9–17. https://doi.org/10.1007/s12020-017-1280-y
Kasasa SC, Soory M (1996) The effect of interleukin-1 (IL-1) on androgen metabolism in human gingival tissue (HGT) and periodontal ligament (PDL). J Clin Periodontol 23(5):419–424. https://doi.org/10.1111/j.1600-051x.1996.tb00568.x
Ojanotko A, Nienstedt W, Harri MP (1980) Metabolism of testosterone by human healthy and inflamed gingiva in vitro. Arch Oral Biol 25(7):481–484. https://doi.org/10.1016/0003-9969(80)90055-2
Vittek J, Rappaport SC, Gordon GG, Munnangi PR, Southren AL (1979) Concentration of circulating hormones and metabolism of androgens by human gingiva. J Periodontol 50(5):254–264. https://doi.org/10.1902/jop.1979.50.5.254
Brusca MI, Verdugo F, Amighini C, Albaina O, Moragues MD (2014) Anabolic steroids affect human periodontal health and microbiota. Clin Oral Investig 18(6):1579–1586. https://doi.org/10.1007/s00784-013-1126-9
Kornman KS, Loesche WJ (1982) Effects of estradiol and progesterone on Bacteroides melaninogenicus and Bacteroides gingivalis. Infect Immun 35(1):256–263. https://doi.org/10.1128/iai.35.1.256-263.1982
Sanz M, van Winkelhoff AJ, Working Group 1 of Seventh European Workshop on Periodontology (2011) Periodontal infections: understanding the complexity--consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol 38(Suppl11):3–6. https://doi.org/10.1111/j.1600-051X.2010.01681.x
Dong Z, Lv W, Zhang C, Chen S (2022) Correlation analysis of gut microbiota and serum metabolome with porphyromonas gingivalis-induced metabolic disorders. Front Cell Infect Microbiol 12:858902. https://doi.org/10.3389/fcimb.2022.858902
Tsuzuno T, Takahashi N, Yamada-Hara M, Yokoji-Takeuchi M, Sulijaya B, Aoki-Nonaka Y et al (2021) Ingestion of porphyromonas gingivalis exacerbates colitis via intestinal epithelial barrier disruption in mice. J Periodontal Res 56(2):275–288. https://doi.org/10.1111/jre.12816
Yamazaki K, Kato T, Tsuboi Y, Miyauchi E, Suda W, Sato K et al (2021) Oral pathobiont-induced changes in gut microbiota aggravate the pathology of nonalcoholic fatty liver disease in mice. Front Immunol 12:766170. https://doi.org/10.3389/fimmu.2021.766170
Graves DT, Oates T, Garlet GP (2011) Review of osteoimmunology and the host response in endodontic and periodontal lesions. J Oral Microbiol 3(1):5304
Khosla S, Atkinson EJ, Dunstan CR, O’Fallon WM (2002) Effect of estrogen versus testosterone on circulating osteoprotegerin and other cytokine levels in normal elderly men. J Clin Endocrinol Metab 87(4):1550–1554. https://doi.org/10.1210/jcem.87.4.8397
Maggio M, Blackford A, Taub D, Carducci M, Ble A, Metter EJ, Braga-Basaria M, Dobs A, Basaria S (2006) Circulating inflammatory cytokine expression in men with prostate cancer undergoing androgen deprivation therapy. J Androl 27(6):725–728. https://doi.org/10.2164/jandrol.106.000141
Yialamas MA, Dwyer AA, Hanley E, Lee H, Pitteloud N, Hayes FJ (2007) Acute sex steroid withdrawal reduces insulin sensitivity in healthy men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 92(11):4254–4259. https://doi.org/10.1210/jc.2007-0454
Gornstein RA, Lapp CA, Bustos-Valdes SM, Zamorano P (1999) Androgens modulate interleukin-6 production by gingival fibroblasts in vitro. J Periodontol 70(6):604–609. https://doi.org/10.1902/jop.1999.70.6.604
Gilliver SC, Ashcroft GS (2007) Sex steroids and cutaneous wound healing: the contrasting influences of estrogens and androgens. Climacteric 10(4):276–288. https://doi.org/10.1080/13697130701456630
Bernard P, Scior T, Do QT (2012) Modulating testosterone pathway: a new strategy to tackle male skin aging? Clin Interv Aging 7:351–361. https://doi.org/10.2147/CIA.S34034
Baggio G, Donazzan S, Monti D, Mari D, Martini S, Gabelli C, Dalla Vestra M, Previato L, Guido M, Pigozzo S, Cortella I, Crepaldi G, Franceschi C (1998) Lipoprotein(a) and lipoprotein profile in healthy centenarians: a reappraisal of vascular risk factors. FASEB J 12(6):433–437. https://doi.org/10.1096/fasebj.12.6.433
Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH Jr, Heimovitz H, Cohen HJ, Wallace R (1999) Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med 106(5):506–512. https://doi.org/10.1016/s0002-9343(99)00066-2
Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ (1999) Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc 47(6):639–646. https://doi.org/10.1111/j.1532-5415.1999.tb01583.x
Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL (2005) The origins of age-related proinflammatory state. Blood 105(6):2294–2299. https://doi.org/10.1182/blood-2004-07-2599
McNerlan SE, Rea IM, Alexander HD (2002) A whole blood method for measurement of intracellular TNF-alpha, IFN-gamma and IL-2 expression in stimulated CD3+ lymphocytes: differences between young and elderly subjects. Exp Gerontol 37(2–3):227–234. https://doi.org/10.1016/s0531-5565(01)00188-7
O’Mahony L, Holland J, Jackson J, Feighery C, Hennessy TP, Mealy K (1998) Quantitative intracellular cytokine measurement: age-related changes in proinflammatory cytokine production. Clin Exp Immunol 113(2):213–219. https://doi.org/10.1046/j.1365-2249.1998.00641.x
Armstrong ME, Alexander HD, Ritchie JL, McMillan SA, Rea IM (2001) Age-related alterations in basal expression and in vitro, tumour necrosis factor alpha mediated, upregulation of CD11b. Gerontology 47(4):180–185. https://doi.org/10.1159/000052795
Bruunsgaard H, Skinhøj P, Pedersen AN, Schroll M, Pedersen BK (2000) Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin Exp Immunol 121(2):255–260. https://doi.org/10.1046/j.1365-2249.2000.01281.x
Bruunsgaard H, Ladelund S, Pedersen AN, Schroll M, Jørgensen T, Pedersen BK (2003) Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clin Exp Immunol 132(1):24–31. https://doi.org/10.1046/j.1365-2249.2003.02137.x
Bruunsgaard H, Andersen-Ranberg K, Hjelmborg J, Pedersen BK, Jeune B (2003) Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am J Med 115(4):278–283. https://doi.org/10.1016/s0002-9343(03)00329-2
Bruunsgaard H, Østergaard L, Andersen-Ranberg K, Jeune B, Pedersen BK (2002) Proinflammatory cytokines, antibodies to Chlamydia pneumoniae and age-associated diseases in Danish centenarians: is there a link? Scand J Infect Dis 34(7):493–499. https://doi.org/10.1080/00365540110080854
Rink L, Cakman I, Kirchner H (1998) Altered cytokine production in the elderly. Mech Ageing Dev 102(2–3):199–209. https://doi.org/10.1016/s0047-6374(97)00153-x
Wieczorowska-Tobis K, Niemir ZI, Podkówka R, Korybalska K, Mossakowska M, Breborowicz A (2006) Can an increased level of circulating IL-8 be a predictor of human longevity? Med Sci Monit 12(3):118–121
Funding
This study was supported by a grant from The Scientific and Technological Research Council of Turkiye (TUBİTAK) (the grant number 2214/A 1059B141600460.)
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the work. Y.Ö.K., R.O., and A.K. desinged study. The clinical study did by Y.Ö.K. The in vitro experiments were by Y.Ö.K. and A.K; and K.K. analyzed the data. Y.Ö.K., E.B., and A.K. contributed to the preparation of the manuscript. All authors gave their final approval and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval
All procedure performed in study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all participants included in the study. This study was approved by Institutional Ethics Committee of the Ataturk University, Faculty of Dentistry. (approval no. 7–02/2016, 02/10/2016).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Özkan Karasu, Y., Orbak, R., Kaşalı, K. et al. Porphyromonas gingivalis enhances the senescence-induced increase of 5-alpha reductase in gingival fibroblasts. Clin Oral Invest 27, 5977–5989 (2023). https://doi.org/10.1007/s00784-023-05211-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-023-05211-y