Abstract
Objective
To investigate the functional changes of PDL fibroblasts in the presence of mechanical force, inflammation, or a combination of force and inflammation.
Materials and methods
Inflammatory supernatants were prepared by inoculating human neutrophils with Porphyromonas gingivalis. Primary human PDL fibroblasts (PDLF), gingival fibroblasts (GFs), and osteoblasts (Saos2) were then exposed to the inflammatory supernatants. Orthodontic force on the PDLFs was simulated by centrifugation. Analyses included cell proliferation, cell viability, cell cycle, and collagen expression, as well as osteoprotegerin (OPG) and receptor activator of nuclear factor kappa-Β ligand (RANKL) expression.
Results
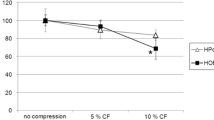
Mechanical force did not affect PDLF viability, but it increased the metabolic rate compared to resting cells. Force application shifted the PDLF cell cycle to the G0/G1 phase, arresting cell proliferation and leading to elevated collagen production, mild OPG level elevation, and robust RANKL level elevation. Including an inflammatory supernatant in the presence of force did not affect PDLF viability, proliferation, or cytokine expression. By contrast, the inflammatory supernatant increased RANKL expression in GFs, but not in Saos2 cells.
Conclusion
Applying mechanical force significantly affects PDLF function. Although inflammation had no effect on PDLF or Saos2 cells, it promoted RANKL expression in GF cells. Within the limitations of the in vitro model, the results suggest that periodontal inflammation and mechanical forces could affect bone catabolism through effects on different cell types, which may culminate in synergistic bone resorption.






Similar content being viewed by others
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
References
Alstad S, Zachrisson BU (1979) Longitudinal study of periodontal condition associated with orthodontic treatment in adolescents. Am J Orthod 76:277–286
Boyd RL, Leggott PJ, Quinn RS, Eakle WS, Chambers D (1989) Periodontal implications of orthodontic treatment in adults with reduced or normal periodontal tissues versus those of adolescents. Am J Orthod Dentofac Orthop:Off Publ Am Assoc Orthod, Constituent Soc, Am Board Orthod 96:191–198
Ong MA, Wang HL, Smith FN (1998) Interrelationship between periodontics and adult orthodontics. J Clin Periodontol 25:271–277
Garat JA, Gordillo ME, Ubios AM (2005) Bone response to different strength orthodontic forces in animals with periodontitis. J Periodontal Res 40:441–445. https://doi.org/10.1111/j.1600-0765.2005.00809.x
Geraci TF, Nevins M, Crossetti HW, Drizen K, Ruben MP (1990) Reattachment of the periodontium after tooth movement into an osseous defect in a monkey. 1. Int J Periodontics Restorative Dent 10:184–197
Gkantidis N, Christou P, Topouzelis N (2010) The orthodontic-periodontic interrelationship in integrated treatment challenges: a systematic review. J Oral Rehabil 37:377–390. https://doi.org/10.1111/j.1365-2842.2010.02068.x
Re S, Corrente G, Abundo R, Cardaropoli D (2000) Orthodontic treatment in periodontally compromised patients: 12-year report. Int J Periodontics Restorative Dent 20:31–39
Zasciurinskiene E, Lindsten R, Slotte C, Bjerklin K (2016) Orthodontic treatment in periodontitis-susceptible subjects: a systematic literature review. Clin Exp Dental Res 2:162–173. https://doi.org/10.1002/cre2.28
Ericsson I, Thilander B, Lindhe J, Okamoto H (1977) The effect of orthodontic tilting movements on the periodontal tissues of infected and non-infected dentitions in dogs. J Clin Periodontol 4:278–293
Wennstrom JL, Stokland BL, Nyman S, Thilander B (1993) Periodontal tissue response to orthodontic movement of teeth with infrabony pockets. Am J Orthod Dentofac Orthop: Off Publ Am Assoc Orthod, Constituent Soc, Am Board Orthod 103:313–319. https://doi.org/10.1016/0889-5406(93)70011-C
Ishihara Y, Tomikawa K, Deguchi T, Honjo T, Suzuki K, Kono T, Kuboki T, Kamioka H, Takashiba S, Yamashiro T (2015) Interdisciplinary orthodontic treatment for a patient with generalized aggressive periodontitis: assessment of IgG antibodies to identify type of periodontitis and correct timing of treatment. Am J Orthod Dentofac Orthop: Off Publ Am Assoc Orthod, Constituent Soc, Am Board Orthod 147:766–780. https://doi.org/10.1016/j.ajodo.2014.09.022
Mathews DP, Kokich VG (1997) Managing treatment for the orthodontic patient with periodontal problems. Sem Orthod 3:21–38
Maddi A, Scannapieco FA (2013) Oral biofilms, oral and periodontal infections, and systemic disease. Am J Dent 26:249–254
Meikle MC (2006) The tissue, cellular, and molecular regulation of orthodontic tooth movement: 100 years after Carl Sandstedt. Eur J Orthod 28:221–240. https://doi.org/10.1093/ejo/cjl001
Hazan-Molina H, Levin L, Einy S, Aizenbud D (2013) Aggressive periodontitis diagnosed during or before orthodontic treatment. Acta Odontol Scand 71:1023–1031. https://doi.org/10.3109/00016357.2012.749515
Kirschneck C, Fanghanel J, Wahlmann U, Wolf M, Roldan JC, Proff P (2017) Interactive effects of periodontitis and orthodontic tooth movement on dental root resorption, tooth movement velocity and alveolar bone loss in a rat model. Ann Anat= Anatomischer Anzeiger : Off Organ Anatomische Gesellschaft 210:32–43. https://doi.org/10.1016/j.aanat.2016.10.004
Shi J, Liu Z, Kawai T, Zhou Y, Han X (2017) Antibiotic administration alleviates the aggravating effect of orthodontic force on ligature-induced experimental periodontitis bone loss in mice. J Periodontal Res 52:725–733. https://doi.org/10.1111/jre.12439
Tanaka Y, Nakayamada S, Okada Y (2005) Osteoblasts and osteoclasts in bone remodeling and inflammation. Curr Drug Targets Inflamm Allergy 4:325–328
Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, Goncalves RB, Valverde P, Dibart S, Li YP, Miranda LA, Ernst CW, Izumi Y, Taubman MA (2006) B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol 169:987–998. https://doi.org/10.2353/ajpath.2006.060180
Kanzaki H, Chiba M, Shimizu Y, Mitani H (2001) Dual regulation of osteoclast differentiation by periodontal ligament cells through RANKL stimulation and OPG inhibition. J Dent Res 80:887–891. https://doi.org/10.1177/00220345010800030801
Baud’huin M, Duplomb L, Teletchea S, Lamoureux F, Ruiz-Velasco C, Maillasson M, Redini F, Heymann MF, Heymann D (2013) Osteoprotegerin: multiple partners for multiple functions. Cytokine Growth Factor Rev 24:401–409. https://doi.org/10.1016/j.cytogfr.2013.06.001
Sordillo EM, Pearse RN (2003) RANK-Fc: a therapeutic antagonist for RANK-L in myeloma. Cancer 97:802–812. https://doi.org/10.1002/cncr.11134
Ferreira CL, Nunes CMM, Bernardo DV, Pedroso JF, Longo M, Santamaria M Jr, Santamaria MP, Jardini MAN (2018) Effect of orthodontic force associated with cigarette smoke inhalation in healthy and diseased periodontium. A histometric and immunohistochemistry analysis in rats. J Periodontal Res 53:924–931. https://doi.org/10.1111/jre.12584
Wang Y, Wang H, Ye Q, Ye J, Xu C, Lin L, Deng H, Hu R (2013) Co-regulation of LPS and tensile strain downregulating osteogenicity via c-fos expression. Life Sci 93:38–43. https://doi.org/10.1016/j.lfs.2013.05.015
Sun C, Liu F, Cen S, Chen L, Wang Y, Sun H, Deng H, Hu R (2017) Tensile strength suppresses the osteogenesis of periodontal ligament cells in inflammatory microenvironments. Mol Med Rep 16:666–672. https://doi.org/10.3892/mmr.2017.6644
Schroder A, Stumpf J, Paddenberg E, Neubert P, Schatz V, Kostler J, Jantsch J, Deschner J, Proff P, Kirschneck C (2021) Effects of mechanical strain on periodontal ligament fibroblasts in presence of Aggregatibacter actinomycetemcomitans lysate. BMC Oral Health 21:405. https://doi.org/10.1186/s12903-021-01761-3
Rath-Deschner B, Nogueira AVB, Memmert S, Nokhbehsaim M, Augusto Cirelli J, Eick S, Miosge N, Kirschneck C, Kesting M, Deschner J, Jager A, Damanaki A (2021) Regulation of anti-apoptotic SOD2 and BIRC3 in periodontal cells and tissues. Int J Mol Sci 22. https://doi.org/10.3390/ijms22020591
Rath-Deschner B, Nogueira AVB, Beisel-Memmert S, Nokhbehsaim M, Eick S, Cirelli JA, Deschner J, Jager A, Damanaki A (2022) Interaction of periodontitis and orthodontic tooth movement-an in vitro and in vivo study. Clin Oral Investig 26:171–181. https://doi.org/10.1007/s00784-021-03988-4
Nokhbehsaim M, Deschner B, Winter J, Reimann S, Bourauel C, Jepsen S, Jager A, Deschner J (2010) Contribution of orthodontic load to inflammation-mediated periodontal destruction. J Orofac Orthop 71:390–402. https://doi.org/10.1007/s00056-010-1031-7
Nogueira AV, Nokhbehsaim M, Eick S, Bourauel C, Jager A, Jepsen S, Rossa C Jr, Deschner J, Cirelli JA (2014) Biomechanical loading modulates proinflammatory and bone resorptive mediators in bacterial-stimulated PDL cells. Mediators Inflamm 2014:425421. https://doi.org/10.1155/2014/425421
Tzach-Nahman R, Nashef R, Fleissig O, Palmon A, Shapira L, Wilensky A, Nussbaum G (2017) Oral fibroblasts modulate the macrophage response to bacterial challenge. Sci Rep 7:11516. https://doi.org/10.1038/s41598-017-11771-3
Collins SJ, Ruscetti FW, Gallagher RE, Gallo RC (1978) Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci USA 75:2458–2462
Polak D, Shmueli A, Brenner T, Shapira L (2018) Oral infection with P. gingivalis exacerbates autoimmune encephalomyelitis. J Periodontol. https://doi.org/10.1002/JPER.17-0531
Redlich M, Palmon A, Zaks B, Geremi E, Rayzman S, Shoshan S (1998) The effect of centrifugal force on the transcription levels of collagen type I and collagenase in cultured canine gingival fibroblasts. Arch Oral Biol 43:313–316
Krishnan V, Davidovitch Z (2006) Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofac Orthop: Off Publ Am Assoc Orthod, Constituent Soc, Am Board Orthod 129(469):e1-32. https://doi.org/10.1016/j.ajodo.2005.10.007
Babes RM, Tofolean IT, Sandu RG, Baran OE, Cosoreanu V, Ilie MT, Duta AI, Ceausescu MC, Ciucur PM, Costache S, Ganea C, Baran I (2018) Simple discrimination of sub-cycling cells by propidium iodide flow cytometric assay in Jurkat cell samples with extensive DNA fragmentation. Cell Cycle 17:766–779. https://doi.org/10.1080/15384101.2018.1426415
Ariffin SHZ, Yamamoto Z, Abidin LZZ, Wahab RMA, Ariffin ZZ (2011) Cellular and molecular changes in orthodontic tooth movement. TheScientificWorldJournal 11:1788–803. https://doi.org/10.1100/2011/761768
Yucel-Lindberg T, Bage T (2013) Inflammatory mediators in the pathogenesis of periodontitis. Expert Rev Mol Med 15:e7. https://doi.org/10.1017/erm.2013.8
Kanzaki H, Chiba M, Shimizu Y, Mitani H (2002) Periodontal ligament cells under mechanical stress induce osteoclastogenesis by receptor activator of nuclear factor kappaB ligand up-regulation via prostaglandin E2 synthesis. J Bone Miner Res: Off J Am Soc Bone Miner Res 17:210–220. https://doi.org/10.1359/jbmr.2002.17.2.210
Yamaguchi M (2009) RANK/RANKL/OPG during orthodontic tooth movement. Orthod Craniofac Res 12:113–119. https://doi.org/10.1111/j.1601-6343.2009.01444.x
Mori T, Miyamoto T, Yoshida H, Asakawa M, Kawasumi M, Kobayashi T, Morioka H, Chiba K, Toyama Y, Yoshimura A (2011) IL-1beta and TNFalpha-initiated IL-6-STAT3 pathway is critical in mediating inflammatory cytokines and RANKL expression in inflammatory arthritis. Int Immunol 23:701–712. https://doi.org/10.1093/intimm/dxr077
Nakashima T, Kobayashi Y, Yamasaki S, Kawakami A, Eguchi K, Sasaki H, Sakai H (2000) Protein expression and functional difference of membrane-bound and soluble receptor activator of NF-kappaB ligand: modulation of the expression by osteotropic factors and cytokines. Biochem Biophys Res Commun 275:768–775. https://doi.org/10.1006/bbrc.2000.3379
Bartold PM (2000) Cantley MD and Haynes DR (2010) Mechanisms and control of pathologic bone loss in periodontitis. Periodontol 53:55–69. https://doi.org/10.1111/j.1600-0757.2010.00347.x
Hormdee D, Nagasawa T, Kiji M, Yashiro R, Kobayashi H, Koshy G, Noguchi K, Nitta H, Ishikawa I (2005) Protein kinase-A-dependent osteoprotegerin production on interleukin-1 stimulation in human gingival fibroblasts is distinct from periodontal ligament fibroblasts. Clin Exp Immunol 142:490–497. https://doi.org/10.1111/j.1365-2249.2005.02937.x
Soames JV, Entwisle DN, Davies RM (1976) The progression of gingivitis to periodontitis in the beagle dog: a histological and morphometric investigation. J Periodontol 47:435–439. https://doi.org/10.1902/jop.1976.47.8.435
Boas Nogueira AV, Chaves de Souza JA, Kim YJ, Damiao de Sousa-Neto M, Chan Cirelli C, Cirelli JA (2013) Orthodontic force increases interleukin-1beta and tumor necrosis factor-alpha expression and alveolar bone loss in periodontitis. J Periodontol 84:1319–1326. https://doi.org/10.1902/jop.2012.120510
Koka S, Reinhardt RA (1997) Periodontal pathogen-related stimulation indicates unique phenotype of primary cultured human fibroblasts from gingiva and periodontal ligament: implications for oral health disease. J Prosthet Dent 77:191–196
Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Morinaga T, Higashio K, Martin TJ, Suda T (2000) Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med 191:275–286. https://doi.org/10.1084/jem.191.2.275
Weitzmann MN, Roggia C, Toraldo G, Weitzmann L, Pacifici R (2002) Increased production of IL-7 uncouples bone formation from bone resorption during estrogen deficiency. J Clin Invest 110:1643–1650. https://doi.org/10.1172/JCI15687
Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397:315–323. https://doi.org/10.1038/16852
Galea GL, Hannuna S, Meakin LB, Delisser PJ, Lanyon LE, Price JS (2015) Quantification of alterations in cortical bone geometry using site specificity software in mouse models of aging and the responses to ovariectomy and altered loading. Front Endocrinol (Lausanne) 6:52. https://doi.org/10.3389/fendo.2015.00052
Author information
Authors and Affiliations
Contributions
T.C. conceived the ideas, collected the data, analyzed the data, and contributed to the writing, revisions, and final approval of the manuscript. Y.I. collected the data, analyzed the data, and contributed to the writing. R.T.N. analyzed the data and contributed to the writing. A.S. analyzed the data and contributed to the writing, revisions, and final approval of the manuscript. L.S. analyzed the data and contributed to the writing, revisions, and final approval of the manuscript. D.P. conceived the ideas, collected the data, analyzed the data, and contributed to the writing, revisions, and final approval of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Experiments involving human tissues were conducted according to the approval of the Helsinki committee of the Hadassah—Hebrew University Medical Center (approval number HMO-12–144-08).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lior Shapira and David Polak have equal contribution to the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chachartchi, T., Itai, Y., Tzach-Nahman, R. et al. Mechanical force application and inflammation induce osteoclastogenesis by independent pathways. Clin Oral Invest 27, 5853–5863 (2023). https://doi.org/10.1007/s00784-023-05196-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-023-05196-8