Abstract
Objectives
The effects of different concentrations of titanium dioxide (TiO2) into 40% hydrogen peroxide (HP) were evaluated as regards the effectiveness of dental color change either associated with activation by polywave LED light or not.
Materials and methods
TiO2 (0, 1, 5, or 10%) was incorporated into HP to be applied during in-office bleaching (3 sessions/40 min each). Polywave LED light (Valo Corded/Ultradent) was applied or not in activation cycles of 15 s (total time of 2 min). The color of 80 third molars separated into groups according to TiO2 concentration and light activation (n = 10) was evaluated at baseline and at time intervals after the 1st, 2nd, and 3rd bleaching sessions.
Results
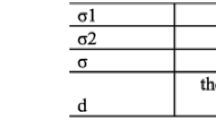
WID value was significantly higher when using HP with 5% TiO2 in the 2nd session than the values in the other groups (p < 0.05). After the 2nd and 3rd sessions, the ΔEab value was significantly higher when activated with light (p < 0.05) for all agents containing TiO2 or not. Zeta potential and pH of the agents were not modified by incorporating TiO2 at the different concentrations.
Conclusions
The 5% TiO2 in the bleaching agent could enhance tooth bleaching, even without light application. Association with polywave LED light potentiated the color change, irrespective of the presence of TiO2 in the bleaching gel.
Clinical significance
HP with 5% TiO2 could lead to a greater tooth bleaching response in the 2nd clinical session, as well as the polywave light can enhance color change.
Similar content being viewed by others
Data Availability
Authors can confirm that all relevant data are included in the article and other supplementary information are available from the corresponding author upon reasonable request.
References
Geus JL, Wambier LM, Kossatz S, Loguercio AD, Reis A (2016) At-home vs in-office bleaching: a systematic review and meta-analysis. Oper Dent 41(4):341–56
Pavicic DK, Kolceg M, Lajnert V, Pavlic A, Brumini M, Spalj S (2018) Changes in quality of life induced by tooth whitening are moderated by perfectionism: a randomized, double-blind, placebo-controlled trial. Int J Prosthodont 31(4):394–96
Mushashe AM, Coelho BS, Garcia PP, Rechia BN, da Cunha LF, Correr GM, Gonzaga CC (2018) Effect of different bleaching protocols on whitening efficiency and enamel superficial microhardness. J Clin Exp Dent 10(8):e772–e775
Komatsu O, Hishida H, Sekino T, Yamamoto K (2014) Application of titanium dioxide nanotubes to tooth whitening. Nanobiomedicine 6(2):63–72
Mondelli R, Rizzante F, Rosa ER, Borges A, Furuse AY, Bombonatti J (2018) Effectiveness of LED/laser irradiation on in-office dental bleaching after three years. Oper Dent 43(1):31–37
Pinto MM, Gonçalves ML, Mota AC, Deana AM, Olivan SR, Bortoletto C, Godoy CH, Vergilio KL, Altavista OM, Motta LJ, Bussadori SK (2017) Controlled clinical trial addressing teeth whitening with hydrogen peroxide in adolescents: a 12-month follow-up. Clinics 72(3):161–170
Tano E, Otsuki M, Kato J, Sadr A, Ikeda M, Tagami J (2012) Effects of 405 nm diode laser on titanium oxide bleaching activation. Photomed Laser Surg 30(11):648–654
Thacker M, Chen YN, Lin CP, Lin FH (2021) Nitrogen-doped titanium dioxide mixed with calcium peroxide and methylcellulose for dental bleaching under visible light activation. Int J Mol Sci 22(7):3759
Martín J, Vildósola P, Bersezio C, Herrera A, Bortolatto J, Saad JR, Oliveira OB Jr, Fernández E (2015) Effectiveness of 6% hydrogen peroxide concentration for tooth bleaching—a double-blind, randomized clinical trial. J Dent 43(8):965–972
Cuppini M, Leitune VCB, Souza M, Alves AK, Samuel SMW, Collares FM (2019) In vitro evaluation of visible light-activated titanium dioxide photocatalysis for in-office dental bleaching. Dent Mater J 38(1):68–74
Sakai K, Kato J, Kurata H, Nakazawa T, Akashi G, Kameyama A et al (2007) The amounts of hydroxyl radicals generated by titanium dioxide and 3.5% hydrogen peroxide under 405-nm diode laser irradiation. Laser Phys 17(8):1062–6
Monteiro NR, Basting RT, Amaral FLBD, França FMG, Turssi CP, Gomes OP, Lisboa Filho PN, Kantovitz KR, Basting RT (2020) Titanium dioxide nanotubes incorporated into bleaching agents: physicochemical characterization and enamel color change. J Appl Oral Sci 24(28):e20190771
Kishi A, Otsuki M, Sadr A, Ikeda M, Tagami J (2011) Effect of light units on tooth bleaching with visible-light activating titanium dioxide photocatalyst. Dent Mater J 30(5):723–729
Lu L, Yoshikawa C, Komatsu O, Hirota Y, Hattori Y, Inoue C (2013) Evaluation of a tooth bleaching system incorporating titanium dioxide. J Osaka Dent Univ 47(2):409–414
Rueggeberg FA, Giannini M, Arrais CAG, Price RBT (2017) Light curing in dentistry and clinical implications: a literature review. Braz Oral Res 31(suppl 1):e61
Soares CJ, Rodrigues MP, Oliveira LRS, Braga SSL, Barcelos LM, Silva GRD, Giannini M, Price RB (2017) An evaluation of the light output from 22 contemporary light curing units. Braz Dent J 28(3):362–71
Soares CJ, Braga SSL, Price RB (2021) Relationship between the cost of 12 light-curing units and their radiant power, emission spectrum, radiant exitance, and beam profile. Oper Dent 46(3):283–92
Arruda LB, Santos CM, Orlandi MO, Schreiner WH, Lisboa-Filho PN (2015) Formation and evolution of TiO2 nanotubes in alkaline synthesis. Ceram Int 41(2):2884–2891
Zha L, Li L, Bao L (2007) Synthesis and colloidal stability of poly(N- isopropylacrylamide) microgels with different ionic groups on their surfaces. J Appl Polym Sci 103(6):3893–3898
Hanaor DAH, Michelazzi M, Leonelli C, Sorrell CC (2012) The effects of carboxylic acids on the aqueous dispersion andelectrophoretic deposition of ZrO2. J Eur Ceram Soc 32:235–244
Faul F, Erdfelder E, Lang AG, Buchner A (2007) GPower 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191
Featherstone JDB, Reilly MM, Shariatti M, Brugler S (1986) Enhancement of remineralization in vitro and in vivo. In: Leach SA (ed) Factors relating to demineralization and remineralization of the teeth. Oxford, IRL Press, p 23–34
Serra MC, Cury JA (1992) The in vitro effect of glass-ionomer cement restoration on enamel subjected to a demineralization and remineralization model. Quintessence Int 23(2):143–147
Torres CR, Crastechini E, Feitosa FA, Pucci CR, Borges AB (2014) Influence of pH on the effectiveness of hydrogen peroxide whitening. Oper Dent 39(6):E261–E268
Ferreira AC, Batista AL, Neto JA, Simões TM, da Silva MG, de Barros DD, Catão JS, de Oliveira TA, Catão MV (2021) Evaluation of dental enamel microproperties after bleaching with 35% hydrogen peroxide and different light sources: an in vitro study. J Clin Exp Dent 13(10):e969–e974
Commission Internationale del clairage (2004)Colorimetry. CIE Central Bureau, Vienna
Paravina RD, Ghinea R, Herrera LJ, Bona AD, Igiel C, Linninger M et al (2015) Color difference thresholds in dentistry. J Esthet Restor Dent 27(Suppl 1):S1-9
Sharma G, Wu W, Dalal EN (2005) The CIEDE2000 color-difference formula: implementation notes, supplementary test data, and mathematical observations. Color Res Appl 30:21
Pérez MM, Ghinea R, Rivas MJ, Yebra A, Ionescu AM, Paravina RD et al (2016) Development of a customized whiteness index for dentistry based on CIELAB color space. Dent Mater 32(3):461–467
Pérez MM, Herrera LJ, Carrillo F, Pecho OE, Dudea D, Gasparik C et al (2019) Whiteness difference thresholds in dentistry. Dent Mater 35(2):292–297
Frysh H, Bowles WH, Baker F, Rivera-Hidalgo F, Guillen G (1995) Effect of pH on hydrogen peroxide bleaching agents. J Esthet Dent 7(3):130–133
Young N, Fairley P, Mohan V, Jumeaux C (2012) A study of hydrogen peroxide chemistry and photochemistry in tea stain solution with relevance to clinical tooth whitening. J Dent 40(Suppl 2):e11–e16
Balladares L, Alegría-Acevedo LF, Montenegro-Arana A, Arana-Gordillo LA, Pulido C, Salazar-Gracez MT, Reis A, Loguercio AD (2019) Effects of pH and application technique of in-office bleaching gels on hydrogen peroxide penetration into the pulp chamber. Oper Dent 44(6):659–67
Acuña ED, Parreiras SO, Favoreto MW, Cruz GP, Gomes A, Borges CP et al (2022) In-office bleaching with a commercial 40% hydrogen peroxide gel modified to have different pHs: color change, surface morphology, and penetration of hydrogen peroxide into the pulp chamber. J Esthet Restor Dent 34(2):322–327
Meireles SS, Heckmann SS, Leida FL, dos Santos Ida S, Della Bona A, Demarco FF (2008) Efficacy and safety of 10% and 16% carbamide peroxide tooth- whitening gels: a randomized clinical trial. Oper Dent 33(6):606–12
Zhang Wx (2003) Nanoscale iron particles for environmental remediation: an overview. J Nanopart Res 5:323–332
Wu L, Zhang J, Watanabe W (2011) Physical and chemical stability of drug nanoparticles. Adv Drug Deliv Rev 63(6):456–469
Mohanraj VJ, Chen Y (2006) Nanoparticles - a review. Trop J Pharm Res 5(1):561–573
Buchalla W, Attin T (2007) External bleaching therapy with activation by heat, light or laser–a systematic review. Dent Mater 23(5):586–596
Alshammery S (2019) Evaluation of light activation on in-office dental bleaching: a systematic review. J Contemp Dent Pract 20(11):1355–1360
Maran BM, Ziegelmann PK, Burey A, de Paris MT, Loguercio AD, Reis A (2019) Different light-activation systems associated with dental bleaching: a systematic review and a network meta-analysis. Clin Oral Investig 23(4):1499–1512
SoutoMaior JR, de Moraes S, Lemos C, Vasconcelos BDE, Montes M, Pellizzer EP (2019) Effectiveness of light sources on in-office dental bleaching: a systematic review and meta-analyses. Oper Dent 44(3):E105–E117
Casado B, Pellizzer EP, SoutoMaior JR, Lemos C, Vasconcelos B, Moraes S (2020) Laser influence on dental sensitivity compared to other light sources used during in-office dental bleaching: systematic review and meta-analysis. Oper Dent 45(6):589–597
Rotstein I, Torek Y, Lewinstein I (1991) Effect of bleaching time and temperature on the radicular penetration of hydrogen peroxide. Endod Dent Traumatol 7(5):196–198
Brugnera AP, Nammour S, Rodrigues JA, Mayer-Santos E, de Freitas PM, Brugnera A Junior, Zanin F (2020) Clinical evaluation of in-office dental bleaching using a violet light-emitted diode. Photobiomodul Photomed Laser Surg 38(2):98–104
Zarpellon DC, Runnacles P, Maucoski C, Coelho U, Rueggeberg FA, Arrais C (2019) Controlling in vivo, human pulp temperature rise caused by LED curing light exposure. Oper Dent 44(3):235–41
Joiner A, Luo W (2017) Tooth colour and whiteness: A review. J Dent 67S:S3–S10
Lilaj B, Dauti R, Agis H, Schmid-Schwap M, Franz A, Kanz F, Moritz A, Schedle A, Cvikl B (2019) Comparison of bleaching products with up to 6% and with more than 6% hydrogen peroxide: whitening efficacy using BI and WID and side effects - an in vitro study. Front Physiol 21(10):919
Author information
Authors and Affiliations
Contributions
Edina Veloso Gonçalves Antunes and Rosanna Tarkany Basting were responsible for developing the experiment, discussion of the results, and writing the paper. Flávia Lucisano Botelho do Amaral, Fabiana Mantovani Gomes França, Kamila Rosamilia Kantovitz, and Cecilia Pedroso Turssi interpreted the results and wrote the paper. Erika Soares Bronze-Uhle and Paulo Noronha Lisboa Filho developed and provided the TiO2. Roberta Tarkany Basting developed the experimental design, wrote the paper, and helped with the discussion.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Antunes, E.V.G., Basting, R.T., do Amaral, F.L.B. et al. Titanium dioxide nanotubes in a hydrogen peroxide-based bleaching agent: physicochemical properties and effectiveness of dental bleaching under the influence of a poliwave led light activation. Clin Oral Invest 27, 1745–1755 (2023). https://doi.org/10.1007/s00784-022-04802-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-022-04802-5