Abstract
Objective
To identify the effect of two chitosan solutions on the release of root dentin matrix proteins and to describe the chemical changes observed following conditioning with chelating agents.
Materials and methods
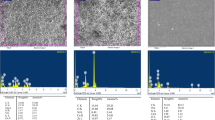
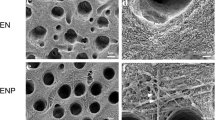
The release of dentin sialoprotein (DSP), transforming growth factor-beta 1 (TGF-β1), vascular endothelial growth factor (VEGF), and platelet-derived growth factor-BB (PDGF-BB) with different chelating agents, including ethylenediaminetetraacetic acid (EDTA), chitosan solution (CS), and nanoparticulate chitosan (CSnp), was investigated. DSP was quantified using an enzyme-linked immunosorbent assay (ELISA). TGF-β1, VEGF, and PDGF-BB were quantified using a cytokine bead panel (CBA). Raman spectroscopy was performed to identify surface chemical changes. Statistical analysis was performed using Kruskal–Wallis test with Mann–Whitney–Wilcoxon rank-sum test (p < 0.05).
Results
TGF-β1, VEGF, and DSP solubilized in all irrigants tested. CSnp showed the highest concentration of DSP. PDGF-BB did not exceed the detection limits. Raman spectroscopy revealed a decrease in the phosphate and carbonate peaks, representing the chelating effect of EDTA, CS, and CSnp. Additionally, CSnp showed the greatest preservation of the amide I and III content.
Conclusion
Proteins can be released from dentin via EDTA, CS, and CSnp conditioning. Raman spectroscopic revealed changes in the inorganic content of the root dentin after chelation. Furthermore, use of CSnp facilitated a preservation of the organic content.
Clinical relevance
Chelation allows the release of proteins, justifying the use of chelating agents in regenerative endodontics. The chitosan–dentin matrix interaction also promotes the protection of the organic content as an additional benefit to its protein releasing effect.






Similar content being viewed by others
References
Xie Z, Shen Z, Zhan P, Yang J, Huang Q, Huang S, Chen L, Lin Z (2021) Functional dental pulp regeneration: basic research and clinical translation. Int J Mol Sci 22:8991. https://doi.org/10.3390/ijms22168991
Elnawam H, Abdelmougod M, Mobarak A, Hussein M, Aboualmakarem H, Girgis M, El Backly R (2022) Regenerative endodontics and minimally invasive dentistry: intertwining paths crossing over into clinical translation. Front Bioeng Biotechnol 8(10):837639. https://doi.org/10.3389/fbioe.2022.837639
Smith JG, Smith AJ, Shelton RM, Cooper PR (2015) Dental pulp cell behavior in biomimetic environments. J Dent Res 94:1552–1559. https://doi.org/10.1177/0022034515599767
Huang X, Li Z, Liu A, Liu X, Guo H, Wu M, Yang X, Han B, Xuan K (2021) Microenvironment influences odontogenic mesenchymal stem cells mediated dental pulp regeneration. Front Physiol 12:656588. https://doi.org/10.3389/fphys.2021.656588
Jeeruphan T, Jantarat J, Yanpiset K, Suwannapan L, Khewsawai P, Hargreaves KM (2012) Mahidol study 1: comparison of radiographic and survival outcomes of immature teeth treated with either regenerative endodontic or apexification methods: a retrospective study. J Endod 38:1330–1336. https://doi.org/10.1016/j.joen.2012.06.028
Jung C, Kim S, Sun T, Cho YB, Song M (2019) Pulp-dentin regeneration: current approaches and challenges. J Tissue Eng 10:2041731418819263. https://doi.org/10.1177/2041731418819263
Zhang X, Li H, Sun J, Luo X, Yang H, Xie L, Yang B, Guo W, Tian W (2017) Cell-derived micro-environment helps dental pulp stem cells promote dental pulp regeneration. Cell Prolif 50:e12361. https://doi.org/10.1111/cpr.12361
Dung SZ, Gregory RL, Li Y, Stookey GK (1995) Effect of lactic acid and proteolytic enzymes on the release of organic matrix components from human root dentin. Caries Res 29:483–489. https://doi.org/10.1159/000262119
Widbiller M, Schweikl H, Bruckmann A, Rosendahl A, Hochmuth E, Lindner SR, Buchalla W, Galler KM (2019) Shotgun proteomics of human dentin with different prefractionation methods. Sci Rep 9:4457. https://doi.org/10.1038/s41598-019-41144-x
Kim JY, Xin X, Moioli EK, Chung J, Lee CH, Chen M, Fu SY, Koch PD, Mao JJ (2010) Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Eng Part A 16:3023–3031. https://doi.org/10.1089/ten.tea.2010.0181
Srisuwan T, Wattanapakkavong K (2021) Direct effect of transforming growth factor-beta 1 (TGF-β1) on human apical papilla cell proliferation and mineralisation. Aust Endod J 48:322–330. https://doi.org/10.1111/aej.12572
Zhao S, Sloan AJ, Murray PE, Lumley PJ, Smith AJ (2000) Ultrastructural localisation of TGF-beta exposure in dentine by chemical treatment. Histochem J 32:489–494. https://doi.org/10.1023/a:1004100518245
Zhang M, Jiang F, Zhang X, Wang S, Jin Y, Zhang W, Jiang X (2017) The effects of platelet-derived growth factor-BB on human dental pulp stem cells mediated dentin-pulp complex regeneration. Stem Cells Transl Med 6:2126–2134. https://doi.org/10.1002/sctm.17-0033
Matsushita K, Motani R, Sakuta T, Yamaguchi N, Koga T, Matsuo K, Nagaoka S, Abeyama K, Maruyama I, Torii M (2000) The role of vascular endothelial growth factor in human dental pulp cells: induction of chemotaxis, proliferation, and differentiation and activation of the AP-1-dependent signaling pathway. J Dent Res 79:1596–1603. https://doi.org/10.1177/00220345000790081201
Yuan GH, Yang GB, Wu LA, Chen Z, Chen S (2010) Potential role of dentin sialoprotein by inducing dental pulp mesenchymal stem cell differentiation and mineralization for dental tissue repair. Dent Hypotheses 1:69–75. https://doi.org/10.5436/j.dehy.2010.1.00012
Wigler R, Kaufman AY, Lin S, Steinbock N, Hazan-Molina H, Torneck CD (2013) Revascularization: a treatment for permanent teeth with necrotic pulp and incomplete root development. J Endod Mar 39(3):319–26
Galler KM, Buchalla W, Hiller KA, Federlin M, Eidt A, Schiefersteiner M, Schmalz G (2015) Influence of root canal disinfectants on growth factor release from dentin. J Endod 41:363–368. https://doi.org/10.1016/j.joen.2014.11.021
Tavares S, Pintor A, Mourão CFAB, Magno M, Montemezzi P, Sacco R, Alves G, Scelza MZ (2021) Effect of different root canal irrigant solutions on the release of dentin-growth factors: a systematic review and meta-analysis. Materials (Basel) 14:5829. https://doi.org/10.3390/ma14195829
Aksel H, Albanyan H, Bosaid F, Azim AA (2020) Dentin conditioning protocol for regenerative endodontic procedures. J Endod 46:1099–1104. https://doi.org/10.1016/j.joen.2020.05.010
Fong D, Duceppe N, Hoemann CD (2017) Mesenchymal stem cell detachment with trace trypsin is superior to EDTA for in vitro chemotaxis and adhesion assays. Biochem Biophys Res Commun 484:656–661. https://doi.org/10.1016/j.bbrc.2017.01.171
Gu LS, Huang XQ, Griffin B, Bergeron BR, Pashley DH, Niu LN, Tay FR (2017) Primum non nocere - the effects of sodium hypochlorite on dentin as used in endodontics. Acta Biomater 61:144–156. https://doi.org/10.1016/j.actbio.2017.08.008
Hashmi A, Zhang X, Kishen A (2019) Impact of dentin substrate modification with chitosan-hydroxyapatite precursor nanocomplexes on sealer penetration and tensile strength. J Endod 45(7):935–942. https://doi.org/10.1016/j.joen.2019.03.021
Wu S, Zhou Y, Yu Y, Zhou X, Du W, Wan M, Fan Y, Zhou X, Xu X, Zheng L (2019) Evaluation of chitosan hydrogel for sustained delivery of VEGF for odontogenic differentiation of dental pulp stem cells. Stem Cells Int 2019:1515040. https://doi.org/10.1155/2019/1515040
Kishen A, Shrestha S, Shrestha A, Cheng C, Goh C (2016) Characterizing the collagen stabilizing effect of crosslinked chitosan nanoparticles against collagenase degradation. Dent Mater 32(8):968–77. https://doi.org/10.1016/j.dental.2016.05.005
El-Hady A, Kamel W, Farid M, Sabry D (2021) Efficacy of chitosan as final irrigating solution on TGF-β1 release from root canal dentin and its effect on dental pulp stem cells response. Al-Azhar Dental J Girls 8(2-A):231–241. https://doi.org/10.21608/adjg.2021.28468.1246
Pang NS, Lee SJ, Kim E, Shin DM, Cho SW, Park W, Zhang X, Jung IY (2014) Effect of EDTA on attachment and differentiation of dental pulp stem cells. J Endod 40(6):811–7. https://doi.org/10.1016/j.joen.2013.09.007
ISO/TS 11405 (2015) Dentistry — testing of adhesion to tooth structure. International Organisation for Standardization: Geneva, Switzerland
Silva PV, Guedes DF, Nakadi FV, Pécora JD, Cruz-Filho AM (2013) Chitosan: a new solution for removal of smear layer after root canal instrumentation. Int Endod J 46:332–338. https://doi.org/10.1111/j.1365-2591.2012.02119.x
de Moura MR, Aouada FA, Avena-Bustillos RJ, McHugh TH, Krochta JM, Mattoso LHC (2009) Improved barrier and mechanical properties of novel hydroxypropyl methylcellulose edible films with chitosan/tripolyphosphate nanoparticles. J Food Eng 92:448–453. https://doi.org/10.1016/j.jfoodeng.2008.12.015
Kassaee M, Hosseini S, Elahi SH (2016) A new nano-chitosan irrigant with superior smear layer removal and penetration. Nanochem Res 1:150–156. https://doi.org/10.7508/NCR.2016.02.002
Khalid M, Bora T, Ghaithi AA, Thukral S, Dutta J (2018) Raman spectroscopy detects changes in bone mineral quality and collagen cross-linkage in Staphylococcus infected human bone. Sci Rep 8:9417. https://doi.org/10.1038/s41598-018-27752-z
Kim SG, Malek M, Sigurdsson A, Lin LM, Kahler B (2018) Regenerative endodontics: a comprehensive review. Int Endod J 51(12):1367–1388. https://doi.org/10.1111/iej.12954
Doğan H, Qalt S (2001) Effects of chelating agents and sodium hypochlorite on mineral content of root dentin. J Endod 27(9):578–80. https://doi.org/10.1097/00004770-200109000-00006
Martinho FC, Leite FRM, Arruda-Vasconcelos R, Louzada LM, Darveau RP, Gomes BPFA (2021) Influence of bacterial profiles in cytokine and clinical features of endodontic disease. J Endod 47:1265–1271. https://doi.org/10.1016/j.joen.2021.04.026
Morgan E, Varro R, Sepulveda H, Ember JA, Apgar J, Wilson J, Lowe L, Chen R, Shivraj L, Agadir A, Campos R, Ernst D, Gaur A (2004) Cytometric bead array: a multiplexed assay platform with applications in various areas of biology. Clin Immunol 110:252–266. https://doi.org/10.1016/j.clim.2003.11.017
Baker SM, Sugars RV, Wendel M, Smith AJ, Waddington RJ, Cooper PR, Sloan AJ (2009) TGF-beta/extracellular matrix interactions in dentin matrix: a role in regulating sequestration and protection of bioactivity. Calcif Tissue Int 85:66–74. https://doi.org/10.1007/s00223-009-9248-4
Yoshiba K, Yoshiba N, Nakamura H, Iwaku M, Ozawa H (1996) Immunolocalization of fibronectin during reparative dentinogenesis in human teeth after pulp capping with calcium hydroxide. J Dent Res 75:1590–1597. https://doi.org/10.1177/00220345960750081101
Usuelli M, Meyer T, Mezzenga R, Mitsi M (2021) VEGF and VEGFR2 bind to similar pH-sensitive sites on fibronectin, exposed by heparin-mediated conformational changes. J Biol Chem 296:100584. https://doi.org/10.1016/j.jbc.2021.100584
Mochizuki M, Güç E, Park AJ, Julier Z, Briquez PS, Kuhn GA, Müller R, Swartz MA, Hubbell JA, Martino MM (2020) Growth factors with enhanced syndecan binding generate tonic signalling and promote tissue healing. Nat Biomed Eng 4:463–475. https://doi.org/10.1038/s41551-019-0469-1
Ferreira LN, Puppin-Rontani RM, Pascon FM (2020) Effect of intracanal medicaments and irrigants on the release of transforming growth factor beta 1 and vascular endothelial growth factor from cervical root dentin. J Endod 46:1616–1622. https://doi.org/10.1016/j.joen.2020.07.034
Khan JA, Hasan A, Dossa S, Ali B (2021) Effect of natural and artificial dentin conditioners on the release of vascular endothelial growth factor. J Endod 47:800–805. https://doi.org/10.1016/j.joen.2021.02.001
Zeng Q, Nguyen S, Zhang H, Chebrolu HP, Alzebdeh D, Badi MA, Kim JR, Ling J, Yang M (2016) Release of growth factors into root canal by irrigations in regenerative endodontics. J Endod 42:1760–1766. https://doi.org/10.1016/j.joen.2016.04.029
Evanko SP, Raines EW, Ross R, Gold LI, Wight TN (1998) Proteoglycan distribution in lesions of atherosclerosis depends on lesion severity, structural characteristics, and the proximity of platelet-derived growth factor and transforming growth factor-beta. Am J Pathol 152:533–546
Goldberg M, Kulkarni AB, Young M, Boskey A (2011) Dentin: structure, composition and mineralization. Front Biosci 3:711–735. https://doi.org/10.2741/e281
Hong S, Li L, Cai W, Jiang B (2018) The potential application of concentrated growth factor in regenerative endodontics. Int Endod J 52(5):646–655. https://doi.org/10.1111/iej.13045
Ratih DN, Sari NI, Santosa P, Kaswati N (2020) Time-dependent effect of chitosan nanoparticles as final irrigation on the apical sealing ability and push-out bond strength of root canal obturation. Int J Dent 2020:8887593. https://doi.org/10.1155/2020/8887593
Atesci AA, Avci CB, Tuglu MI, Ozates Ay NP, Eronat AC (2020) Effect of different dentin conditioning agents on growth factor release, mesenchymal stem cell attachment and morphology. J Endod 46:200–208. https://doi.org/10.1016/j.joen.2019.10.033
Mohammadi Z, Shalavi S, Jafarzadeh H (2013) Ethylenediaminetetraacetic acid in endodontics. Eur J Dent 7:S135–S142. https://doi.org/10.4103/1305-7456.119091
Fadeeva IV, Barinov SM, Fedotov AY, Komlev VS (2011) Interactions of calcium phosphates with chitosan. Dokl Chem 441(2):387. https://doi.org/10.1134/S0012500811120044
Del Carpio-Perochena A, Bramante CM, Duarte MA, de Moura MR, Aouada FA, Kishen A (2015) Chelating and antibacterial properties of chitosan nanoparticles on dentin. Restor Dent Endod 40:195–201. https://doi.org/10.5395/rde.2015.40.3.195
Hashmi A, Zhang X, Kishen A (2019) Impact of dentin substrate modification with chitosan-hydroxyapatite precursor nanocomplexes on sealer penetration and tensile strength. J Endod 45(7):935–942. https://doi.org/10.1016/j.joen.2019.03.021
Kishen A, Shi Z, Shrestha A, Neoh KG (2008) An investigation on the antibacterial and antibiofilm efficacy of cationic nanoparticulates for root canal disinfection. J Endod 34(12):1515–1520. https://doi.org/10.1016/j.joen.2008.08.035
Funding
This research was funded by the Universidad Nacional de Colombia through the Convocatoria para el fortalecimiento de alianzas interdisciplinarias de investigación y creación artística sede Bogotá 2018, Project Code: 41999.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures were carried out in accordance with the ethical standards of the ethics and research committee of the Faculty of Dentistry of the National University of Colombia, Code #CIEFO-335–19, and with the Helsinki declaration, 1964, with its respective modifications. All participants provided informed consent for the acquisition and use of the experimental samples.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Quijano-Guauque, S., Bernal-Cepeda, L.J., Delgado, F.G. et al. Effect of chitosan irrigant solutions on the release of bioactive proteins from root dentin. Clin Oral Invest 27, 691–703 (2023). https://doi.org/10.1007/s00784-022-04787-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-022-04787-1