Abstract
Objective
This systematic review assesses the prevalence of microbial complexes in endodontic-periodontal lesion.
Materials and methods
Nine databases were searched through August 2020. Experts were consulted to indicate additional studies. Studies were blindly selected by two reviewers based on pre-defined eligibility criteria. Studies that evaluated the prevalence of microbial orange and red complexes among patients with endodontic-periodontal lesion were considered eligible. Risk of bias was assessed using the Joanna Briggs Institute Critical Appraisal Checklist for Studies Reporting Prevalence Data.
Results
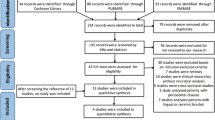
From 572 articles found on all databases, 11 clinical studies were finally included. The following microorganisms were investigated: P. gingivalis, T. forsythia, T. denticola, F. nucleatum, F. periodonticum, P. micra, P. intermedia, P. nigrescens, S. constellatus, C. gracilis, C. rectus, C. showae and E. nodatum. Considering the orange complex, P. micra, E. nodatum and S. constellatus were prevalent in both root canal and periodontal pockets. P. gingivalis and T. forsythia belonging to the red complex were prevalent only in periodontal pockets. The red complex microorganisms were not found very frequently in root canal.
Conclusions
There is a similarity between the microbiome of root canal and periodontal pockets, with prevalence of the three microorganisms of the orange complex. Two microorganisms from the red complex were prevalent only in periodontal pockets.
Clinical relevance
The prevalence of specific microorganisms in endodontic-periodontal lesion is important to understand the microbiological profile of the patients involved and to correlate it with possible clinical and repair conditions of this pathology.

Similar content being viewed by others
References
Simon J, Glick DH, Frank AL (2013) In Remembrance of James H S Simon The relationship of endodontic–periodontic lesions. J Endod 39(5):41–46. https://doi.org/10.1016/j.joen.2013.02.006
Rotstein I (2017) Interaction between endodontics and periodontics Periodontol 2000(74):11–39. https://doi.org/10.1111/prd.12188
Das AC, Sahoo SK, Parihar AS, Bhardwaj SS, Babaji P, Varghese JG (2020) Evaluation of role of periodontal pathogens in endodontic periodontal diseases. J Family Med Prim Care 9(1):239–242. https://doi.org/10.4103/jfmpc.jfmpc_725_19
Herrera D, Retamal-Valdes B, Alonso B, Feres M (2018) Acute periodontal lesions (periodontal abscesses and necrotizing periodontal diseases) and endo-periodontal lesions. J Periodontol 89:S85–S102. https://doi.org/10.1002/JPER.16-0642
Li H, Guan R, Sun J, Hou B (2014) Bacteria community study of combined periodontal-endodontic lesions using denaturing gradient gel electrophoresis and sequencing analysis. J Periodontol 85(10):1442–1449. https://doi.org/10.1902/jop.2014.130572
Gomes BP, Berber VB, Kokaras AS, Chen T, Paster BJ (2015) Microbiomes of Endodontic-Periodontal Lesions before and after Chemomechanical Preparation. J Endod 41(12):1975–1984. https://doi.org/10.1016/j.joen.2015.08.022
Zhang Y, Wang X, Li H, Ni C, Du Z, Yan F (2018) Human oral microbiota and its modulation for oral health. Biomed Pharmacother 99:883–893. https://doi.org/10.1016/j.biopha.2018.01.146
Didilescu AC, Rusu D, Anghel A, Nica L, Iliescu A, Greabu M, Bancescu G, Stratul SI (2012) Investigation of six selected bacterial species in endo-periodontal lesions. Int Endod J 45(3):282–293. https://doi.org/10.1111/j.1365-2591.2011.01974.x
Xia M, Qi Q (2013) Bacterial analysis of combined periodontal-endodontic lesions by polymerase chain reaction-denaturing gradient gel electrophoresis. J Oral Sci 55(4):287–291. https://doi.org/10.2334/josnusd.55.287
Kipioti A, Nakou M, Legakis N, Mitsis F (1984) Microbiological findings of infected root canals and adjacent periodontal pockets in teeth with advanced periodontitis. Oral Surg Oral Med Oral Pathol 58:213–220. https://doi.org/10.1016/0030-4220(84)90139-7
Kurihara H, Kobayashi Y, Francisco IA, Isoshima O, Nagai A, Murayama Y (1995) A microbiological and immunological study of endodontic-periodontic lesions. J Endod 21(12):617–621. https://doi.org/10.1016/S0099-2399(06)81115-5
Simon JH, Glick DH, Frank AL (1972) The relationship endodontic-periodontic lesions. J Periodontol 43(4):202–208. https://doi.org/10.1902/jop.1972.43.4.202
Pereira CV, Stipp RN, Fonseca DC, Pereira LJ, Höfling JF (2011) Detection and clonal analysis of anaerobic bacteria associated to endodontic-periodontal lesions. J Periodontol 82(12):1767–1775. https://doi.org/10.1902/jop.2011.110063
Duque TM, Prado M, Herrera DR, Gomes BPFA (2018) Periodontal and endodontic infectious/inflammatory profile in primary periodontal lesions with secondary endodontic involvement after a calcium hydroxide-based intracanal medication. Clin Oral Investig 23(1):53–63. https://doi.org/10.1007/s00784-018-2401-6
Rovai EDS, Matos FS, Kerbauy WD, Cardoso FGR, Martinho FC, Oliveira LD, Valera MC, Carvalho AT (2019) Microbial profile and endotoxin levels in primary periodontal lesions with secondary endodontic involvement. Braz Dent J 30(4):356–362. https://doi.org/10.1590/0103-6440201902471
Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr (1998) Microbial complexes in subgingival plaque. J Clin Periodontol 25:134–144. https://doi.org/10.1111/j.1600-051x.1998.tb02419.x
Pinto G, Silva MD, Peddey M, Sillankorva S (2016) Azeredo J (2016) The role of bacteriophages in periodontal health and disease. Future Microbiol 11:1359–1369. https://doi.org/10.2217/fmb-2016-0081
Harvey JD (2017) Periodontal Microbiology. Dent Clin North Am 61(2):253–269. https://doi.org/10.1016/j.cden.2016.11.005
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. The BMJ 350:g7647. https://doi.org/10.1136/bmj.g7647
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4(1):1. https://doi.org/10.1186/2046-4053-4-1
Booth A, Clarke M, Ghersi D, Moher D, Petticrew M, Stewart L (2011) An international registry of systematic-review protocols. Lancet (London, England) 377(9760):108–109. https://doi.org/10.1016/s0140-6736(10)60903-8
Higgins JPT, Green S (2011) Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Moola S MZ, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Qureshi R, Mattis P, Lisy K, Mu P-F (2017) Chapter 7: systematic reviews of etiology and risk. Joanna Briggs Institute Reviewer's Manual.
Baumgartner JC, Falkler WA Jr, Bernie RS, Suzuki JB (1992) Serum IgG reactive with oral anaerobic microorganisms associated with infections of endodontic origin. Oral Microbiol Immunol 7(2):106–110. https://doi.org/10.1111/j.1399-302x.1992.tb00518.x
Geibel MA, Schu B, Callaway AS, Gleissner C, Willershausen B (2005) Polymerase chain reaction-based simultaneous detection of selected bacterial species associated with closed periapical lesions. Eur J Med Res 10(8):333–338
Gomes BP, Jacinto RC, Pinheiro ET, Sousa ELR, Zaia AA, Ferraz CCR, Souza-Filho FJ (2006) Molecular analysis of Filifactor alocis, Tannerella forsythia, and treponema denticola associated with primary endodontic infections and failed endodontic treatment. J Endod 32(10):937–940. https://doi.org/10.1016/j.joen.2006.05.003
Moraes SR, Siqueira JF Jr, Colombo AP, Rôças IN, Ferreira MC, Domingues RM (2002) Comparison of the effectiveness of bacterial culture, 16S rDNA directed polymerase chain reaction, and checkerboard DNA-dNA hybridization for detection of Fusobacterium nucleatum in endodontic infections. J Endod 28(2):86–89. https://doi.org/10.1097/00004770-200202000-00009
Muniz FWMG, Montagner F, Jacinto RC, Rösing CK, Gomes BPFA (2018) Correlation between crestal alveolar bone loss with intracanal bacteria and apical lesion area in necrotic teeth. Arch Oral Biol 95:1–6. https://doi.org/10.1016/j.archoralbio.2018.07.007
Rôças IN, Siqueira JF Jr, Andrade AF, Uzeda M (2003) Oral treponemes in primary root canal infections as detected by nested PCR. Int Endod J 36(1):20–26. https://doi.org/10.1046/j.0143-2885.2003.00607.x
Sassone LM, Fidel RA, Faveri M, Figueiredo L, Fidel SR, Feres M (2012) A microbiological profile of unexposed and exposed pulp space of primary endodontic infections by checkerboard DNA-DNA hybridization. J Endod 38(7):889–893. https://doi.org/10.1016/j.joen.2012.03.021
Siqueira JF Jr, Rôças IN (2003) Campylobacter gracilis and Campylobacter rectus in primary endodontic infections. Int Endod J 36(3):174–180. https://doi.org/10.1046/j.1365-2591.2003.00636.x
Siqueira JF, Rôças IN (2003) Positive and negative bacterial associations involving Dialister pneumosintes in primary endodontic infections. J Endod 29(7):438–441. https://doi.org/10.1097/00004770-200307000-00003
Siqueira JF Jr, Rôças IN, Andrade AF, de Uzeda M (2003) Peptostreptococcus micros in primary endodontic infections as detected by 16S rDNA-based polymerase chain reaction. J Endod 29(2):111–113. https://doi.org/10.1097/00004770-200302000-00006
Siqueira JF, Jung IY, Rôças IN, Lee CY (2005) Differences in prevalence of selected bacterial species in primary endodontic infections from two distinct geographic locations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 99(5):641–647. https://doi.org/10.1016/j.tripleo.2004.07.009
Caton JG, Armitage G, Berglundh T, Chapple ILC, Jepsen S, Kornman KS, Mealey BL, Papapanou PN, Sanz M, Tonetti MS (2018) A new classification scheme for periodontal and peri-implant diseases and conditions - introduction and key changes from the 1999 classification. J Clin Periodontol 45(20):S1–S8. https://doi.org/10.1111/jcpe.12935
Siqueira JF Jr, Rôças IN (2017) The oral microbiota in health and disease: an overview of molecular findings. Methods Mol Biol 1537:127–138. https://doi.org/10.1007/978-1-4939-6685-1_7
Berne C, Ellison CK, Ducret A, Brun YV (2018) Bacterial adhesion at the single-cell level. Nat Rev Microbiol 16(10):616–627. https://doi.org/10.1038/s41579-018-0057-5
Koo H, Allanb RN, Howlind RP, Hall-Stoodleye L, Stoodleye P (2017) Targeting microbial biofilms: current and prospective therapeutic strategies. Nat Rev Microbiol 15(12):740–755. https://doi.org/10.1038/nrmicro.2017.99
Lamont RJ, Hajishengallis KH, G, (2018) The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol 16(12):745–759. https://doi.org/10.1038/s41579-018-0089-x
Socransky SS, Haffajee AD (2005) Periodontal microbial ecology Periodontol 2000(38):135–187. https://doi.org/10.1111/j.1600-0757.2005.00107.x
Haffajee AD, Socransky SS, Patel MR, Song X (2008) Microbial complexes in supragingival plaque. Oral Microbiol Immunol 23(3):196–205. https://doi.org/10.1111/j.1399-302X.2007.00411.x
Carda C, Peydró A (2006) Ultrastructural patterns of human dentinal tubules, odontoblasts processes and nerve fibres. Tissue Cell 38(2):141–150. https://doi.org/10.1016/j.tice.2006.01.002
Abarca J, Zaror C, Monardes H, Hermosilla V, Muñoz C, Cantin M (2014) Morphology of the physiological apical foramen in maxillary and mandibular first molars. Int J Morphol 32(2):671–677. https://doi.org/10.4067/S0717-95022014000200048
Whiley RA, Hall LM, Hardie JM, Beighton D (1999) A study of small-colony, beta-haemolytic, Lancefield group C streptococci within the anginosus group: description of Streptococcus constellatus subsp. pharyngis subsp. nov., associated with the human throat and pharyngitis. Int J Syst Bacteriol 49(4):1443–449. https://doi.org/10.1099/00207713-49-4-1443
Whiley RA, Beighton D, Winstanley TG, Fraser HY, Hardie JM (1992) Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (the Streptococcus milleri group): association with different body sites and clinical infections. J Clin Microbiol 30(1):243–244. https://doi.org/10.1128/JCM.30.1.243-244.1992
Asam D, Spellerberg B (2014) Molecular pathogenicity of Streptococcus anginosus. Mol Oral Microbiol 29(4):145–155. https://doi.org/10.1111/omi.12056
Olson AB, Kent H, Sibley CD, Grinwis ME, Mabon P, Ouellette C, Tyson S, Graham M, Tyler SD, Van Domselaar G, Surette MG, Corbett CR (2013) Phylogenetic relationship and virulence inference of Streptococcus anginosus Group: curated annotation and whole-genome comparative analysis support distinct species designation. BMC Genomics 17(14):895. https://doi.org/10.1186/1471-2164-14-895
Imlay JA (2003) Pathways of oxidative damage. Annu Rev Microbiol 57:395–418. https://doi.org/10.1146/annurev.micro.57.030502.090938
Toliver-Kinsky T, Cui W, Törö G, Lee SJ, Shatalin K, Nudler E, Szabo C (2018) H2S, a bacterial defense mechanism against the host immune response. Infect Immun 87(1):e00272-18. https://doi.org/10.1128/IAI.00272-18 (19)
Rahman MA, Glasgow JN, Nadeem S, Reddy VP, Sevalkar RR, Lancaster JR Jr, Steyn AJC (2020) The role of host-generated H2S in microbial pathogenesis: new perspectives on tuberculosis. Front Cell Infect Microbiol 10(10):586923. https://doi.org/10.3389/fcimb.2020.586923
Tindall BJ, Euzéby JP (2006) Proposal of Parvimonas gen. nov. and Quatrionicoccus gen. nov. as replacements for the illegitimate, prokaryotic, generic names Micromonas Murdoch and Shah 2000 and Quadricoccus Maszenan et al 2002, respectively. Int J Syst Evol Microbiol 56(11):2711–2713. https://doi.org/10.1099/ijs.0.64338-0
Watanabe T, Hara Y, Yoshimi Y, Fujita Y, Yokoe M, Noguchi Y (2020) Clinical characteristics of bloodstream infection by Parvimonas micra: retrospective case series and literature review. BMC Infect Dis 20(1):578. https://doi.org/10.1186/s12879-020-05305-y
Tanabe S, Bodet C, Grenier D (2007) Peptostreptococcus micros cell wall elicits a pro-inflammatory response in human macrophages. J Endotoxin Res 13(4):219–226. https://doi.org/10.1177/0968051907081869
Gulabivala K, Ng Y-L (2014) Biological and clinical rationale for root-canal treatment and management of its failure. In: Gulabivala K (ed) Endodontics, 4th edn. Mosby, Yuan-Ling Ng, pp 43–92
Kremer BH, Van-Steenbergen TJ (2000) Peptostreptococcus micros coaggregates with Fusobacterium nucleatum and non-encapsulated Porphyromonas gingivalis. FEMS Microbiol Lett 182(1):57–62. https://doi.org/10.1111/j.1574-6968.2000.tb08873.x
Akashi M, Tanaka K, Kusumoto J, Furudoi S, Hosoda K, Komori T (2017) Brain abscess potentially resulting from odontogenic focus: report of three cases and a literature review. J Maxillofac Oral Surg 16(1):58–64. https://doi.org/10.1007/s12663-016-0915-5
Holdeman LV, Cato EP, Burmeister JA, Moore WEC (1980) Descriptions of Eubacteriumtimidum sp. nov., Eubacteriumbrachy sp. nov., and Eubacteriumnodatum sp. nov. Isolated from Human Periodontitis. Int J Syst Evol Microbiol 30(1):163–169. https://doi.org/10.1099/00207713-30-1-163
Lalla E, Kaplan S, Chang SM, Roth GA, Celenti R, Hinckley K, Greenberg E, Papapanou PN (2006) Periodontal infection profiles in type 1 diabetes. J Clin Periodontol 33(12):855–862. https://doi.org/10.1111/j.1600-051X.2006.00996.x
Abusleme L, Dupuy AK, Dutzan N, Silva N, Burleson JA, Strausbaugh LD, Gamonal J, Diaz PI (2013) The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J 7(5):1016–1025. https://doi.org/10.1038/ismej.2012.174
Feres M, Bernal M, Matarazzo F, Faveri M, Duarte PM, Figueiredo LC (2015) Subgingival bacterial recolonization after scaling and root planning in smokers with chronic periodontitis. Aust Dent J 60(2):225–232. https://doi.org/10.1111/adj.12225
Kalala-Kazadi E, Sekele-Issouradi JP, Bolenge-Ileboso J, Lassere JF, Mantshumba-Milolo A, Ntumba-Mulumba H, Brecx MC (2018) Periopathogenic bacteria in dental plaque of Congolese patients with periodontitis: a pilot study. J Clin Exp Dent 10(3):e232-236. https://doi.org/10.4317/jced.54613
Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshamanan A, Wade WG (2010) The human oral microbiome. J Bacteriol 192(19):5003–5017. https://doi.org/10.1128/JB.00542-10
Alwaeli AJJ (2018) Anaerobic bacteria associated with periodontitis. Intechopen 19:32. https://doi.org/10.5772/intechopen.76352.cap3
Lamont RJ, Koo H, Hajishengallis G (2018) The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol 16(12):745–759. https://doi.org/10.1038/s41579-018-0089-x
Widmer C, Skutas J, Easson C, Lopez JV, Torneck C, Flax M, Sayin TC (2018) Culture-independent characterization of the microbiome of healthy pulp. J Endod 44(7):1132-1139.e2. https://doi.org/10.1016/j.joen.2018.03.009
Fouts DE, Pieper R, Szpakowski S, Pohl H, Knoblach S, Suh MJ, Huang ST, Ljungberg I, Sprague BM, Lucas SK, Torralba M, Nelson KE, Groah SL (2012) Integrated next-generation sequencing Of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic in neuropathic bladder associated with spinal cord injury. J Transl Med 28(10):174. https://doi.org/10.1186/1479-5876-10-174
Mombelli A (2000) (2018) Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontol 76(1):85–96. https://doi.org/10.1111/prd.12147
Genco RJ (2000) Sanz M (2020) Clinical and public health implications of periodontal and systemic diseases: an overview. Periodontol 83(1):7–13. https://doi.org/10.1111/prd.12344
Lira-Junior R, Åkerman S, Klinge B, Boström EA, Gustafsson A (2018) Salivary microbial profiles in relation to age, periodontal, and systemic diseases. PLoS ONE 13(3):e0189374. https://doi.org/10.1371/journal.pone.0189374
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Informed consent
For this type of study, formal consent is not required.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1
Appendix 2
Rights and permissions
About this article
Cite this article
Gambin, D.J., Vitali, F.C., De Carli, J.P. et al. Prevalence of red and orange microbial complexes in endodontic-periodontal lesions: a systematic review and meta-analysis. Clin Oral Invest 25, 6533–6546 (2021). https://doi.org/10.1007/s00784-021-04164-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-021-04164-4