Abstract
Objectives
The purpose of this experimental in vivo investigation was to evaluate the influence of modifying the implant surface by adding a monolayer of multi-phosphonate molecules on the development of experimental peri-implantitis.
Material and methods
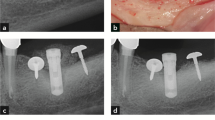
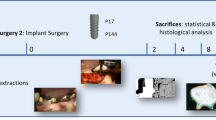
Eight beagle dogs received 5 tests and 5 control implants each following a split-mouth design 3 months after premolar and molar extraction. On the most mesial implant of each side, a 3-mm buccal dehiscence was artificially created. Experimental peri-implantitis was induced by silk ligatures over a 4-month period; after ligature removal, peri-implantitis was left to progress for another 4 months without plaque control. Clinical, histological, and radiographic outcomes were evaluated.
Results
Radiographically, both implant groups showed a similar bone loss (BL) at the end of the induction and progression phases. BL measured on the histological sections of the test and control groups was 3.14 ± 0.42 mm and 3.26 ± 0.28 mm, respectively; the difference was not statistically significant (p > 0.05). The remaining buccal bone to implant contact (bBIC) percentage of the test and control groups was 59.38 ± 18.62 and 47.44 ± 20.46%, respectively; the difference, however, was not statistically significant (p > 0.05). Bone loss observed at dehiscent sites compared to non-dehiscent ones showed no statistically significant difference (p > 0.05).
Conclusions
Addition of a monophosphonate layer to a moderately rough implant surface did not affect development of experimental peri-implantitis.
Clinical relevance
Influence of implant surface on peri-implantitis may condition implant selection by the clinician, especially on patients with disease risk factors. In that sense, monophosphate layer implants do not show higher peri-implantitis risk than control implants.





Similar content being viewed by others
References
Albrektsson T, Branemark PI, Hansson HA, Lindstrom J (1981) Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand 52:155–170. https://doi.org/10.3109/17453678108991776
Schroeder A, van der Zypen E, Stich H, Sutter F (1981) The reactions of bone, connective tissue, and epithelium to endosteal implants with titanium-sprayed surfaces. J Maxillofac Surg 9:15–25. https://doi.org/10.1016/s0301-0503(81)80007-0
Wennerberg A, Albrektsson T, Andersson B (1996) Bone tissue response to commercially pure titanium implants blasted with fine and coarse particles of aluminum oxide. Int J Oral Maxillofac Implants 11:38–45
Vignoletti F, Johansson C, Albrektsson T, De Sanctis M, San Roman F, Sanz M (2009) Early healing of implants placed into fresh extraction sockets: an experimental study in the beagle dog. De novo bone formation. J Clin Periodontol 36:265–277. https://doi.org/10.1111/j.1600-051X.2008.01363.x
Esposito M, Coulthard P, Thomsen P, Worthington HV (2005) The role of implant surface modifications, shape and material on the success of osseointegrated dental implants. A Cochrane systematic review. Eur J Prosthodont Restor Dent 13:15–31
Rossi F, Lang NP, De Santis E, Morelli F, Favero G, Botticelli D (2014) Bone-healing pattern at the surface of titanium implants: an experimental study in the dog. Clin Oral Implants Res 25:124–131. https://doi.org/10.1111/clr.12097
Shah FA, Nilson B, Branemark R, Thomsen P, Palmquist A (2014) The bone-implant interface-nanoscale analysis of clinically retrieved dental implants. Nanomedicine 10:1729–1737. https://doi.org/10.1016/j.nano.2014.05.015
Viornery C, Guenther HL, Aronsson BO, Pechy P, Descouts P, Gratzel M (2002) Osteoblast culture on polished titanium disks modified with phosphonic acids. J Biomed Mater Res 62:149–155. https://doi.org/10.1002/jbm.10205
von Salis-Soglio M, Stubinger S, Sidler M, Klein K, Ferguson SJ, Kampf K, Zlinszky K, Buchini S, Curno R, Pechy P, Aronsson BO, von Rechenberg B (2014) A novel multi-phosphonate surface treatment of titanium dental implants: a study in sheep. J Funct Biomater 5:135–157. https://doi.org/10.3390/jfb5030135
Esposito M, Dojcinovic I, Buchini S, Pechy P, Aronsson BO (2017) Safety and efficacy of a biomimetic monolayer of permanently bound multiphosphonic acid molecules on dental implants: 3 years post-loading results from a pilot quadruple-blinded randomised controlled trial. Eur J Oral Implantol 10:43–54
Schwarz F, Derks J, Monje A, Wang HL (2018) Peri-implantitis. J Clin Periodontol 45(Suppl 20):S246–S266. https://doi.org/10.1111/jcpe.12954
Dreyer H, Grischke J, Tiede C, Eberhard J, Schweitzer A, Toikkanen SE, Glockner S, Krause G, Stiesch M (2018) Epidemiology and risk factors of peri-implantitis: a systematic review. J Periodontal Res 53:657–681. https://doi.org/10.1111/jre.12562
Albouy JP, Abrahamsson I, Berglundh T (2012) Spontaneous progression of experimental peri-implantitis at implants with different surface characteristics: an experimental study in dogs. J Clin Periodontol 39:182–187. https://doi.org/10.1111/j.1600-051X.2011.01820.x
Carcuac O, Abrahamsson I, Derks J, Petzold M, Berglundh T (2020) Spontaneous progression of experimental peri-implantitis in augmented and pristine bone: a pre-clinical in vivo study. Clin Oral Implants Res 31:192–200. https://doi.org/10.1111/clr.13564
Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, Group NCRRGW (2010) Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160:1577–1579. https://doi.org/10.1111/j.1476-5381.2010.00872.x
Sanz-Esporrin J, Blanco J, Sanz-Casado JV, Munoz F, Sanz M (2019) The adjunctive effect of rhBMP-2 on the regeneration of peri-implant bone defects after experimental peri-implantitis. Clin Oral Implants Res 30:1209–1219. https://doi.org/10.1111/clr.13534
Lindhe J, Berglundh T, Ericsson I, Liljenberg B, Marinello C (1992) Experimental breakdown of peri-implant and periodontal tissues. A study in the beagle dog. Clin Oral Implants Res 3:9–16. https://doi.org/10.1034/j.1600-0501.1992.030102.x
Donath K, Breuner G (1982) A method for the study of undecalcified bones and teeth with attached soft tissues. The Sage-Schliff (sawing and grinding) technique. J Oral Pathol 11:318–326. https://doi.org/10.1111/j.1600-0714.1982.tb00172.x
Jeno L, Geza L (1975) A simple differential staining method for semi-thin sections of ossifying cartilage and bone tissues embedded in epoxy resin. Mikroskopie 31:1–4
Berglundh T, Lindhe J, Jonsson K, Ericsson I (1994) The topography of the vascular systems in the periodontal and peri-implant tissues in the dog. J Clin Periodontol 21:189–193. https://doi.org/10.1111/j.1600-051x.1994.tb00302.x
Carcuac O, Abrahamsson I, Albouy JP, Linder E, Larsson L, Berglundh T (2013) Experimental periodontitis and peri-implantitis in dogs. Clin Oral Implants Res 24:363–371. https://doi.org/10.1111/clr.12067
Martins MC, Abi-Rached RS, Shibli JA, Araujo MW, Marcantonio E Jr (2004) Experimental peri-implant tissue breakdown around different dental implant surfaces: clinical and radiographic evaluation in dogs. Int J Oral Maxillofac Implants 19:839–848
Tillmanns HW, Hermann JS, Tiffee JC, Burgess AV, Meffert RM (1998) Evaluation of three different dental implants in ligature-induced peri-implantitis in the beagle dog. Part II. Histology and microbiology. Int J Oral Maxillofac Implants 13:59–68
Madi M, Zakaria O, Noritake K, Fuji M, Kasugai S (2013) Peri-implantitis progression around thin sputtered hydroxyapatite-coated implants: clinical and radiographic evaluation in dogs. Int J Oral Maxillofac Implants 28:701–709. https://doi.org/10.11607/jomi.2891
Godoy-Gallardo M, Manzanares-Cespedes MC, Sevilla P, Nart J, Manzanares N, Manero JM, Gil FJ, Boyd SK, Rodriguez D (2016) Evaluation of bone loss in antibacterial coated dental implants: an experimental study in dogs. Mater Sci Eng C Mater Biol Appl 69:538–545. https://doi.org/10.1016/j.msec.2016.07.020
Lopez-Piriz R, Sola-Linares E, Granizo JJ, Diaz-Guemes I, Enciso S, Bartolome JF, Cabal B, Esteban-Tejeda L, Torrecillas R, Moya JS (2012) Radiologic evaluation of bone loss at implants with biocide coated titanium abutments: a study in the dog. PLoS One 7:e52861. https://doi.org/10.1371/journal.pone.0052861
Blanco J, Pico A, Caneiro L, Novoa L, Batalla P, Martin-Lancharro P (2018) Effect of abutment height on interproximal implant bone level in the early healing: a randomized clinical trial. Clin Oral Implants Res 29:108–117. https://doi.org/10.1111/clr.13108
Galindo-Moreno P, Fernandez-Jimenez A, O’Valle F, Monje A, Silvestre FJ, Juodzbalys G, Sanchez-Fernandez E, Catena A (2015) Influence of the crown-implant connection on the preservation of peri-implant bone: a retrospective multifactorial analysis. Int J Oral Maxillofac Implants 30:384–390. https://doi.org/10.11607/jomi.3804
Monje A, Pommer B (2015) The concept of platform switching to preserve peri-implant bone level: assessment of methodologic quality of systematic reviews. Int J Oral Maxillofac Implants 30:1084–1092. https://doi.org/10.11607/jomi.4103
Jung RE, Herzog M, Wolleb K, Ramel CF, Thoma DS, Hammerle CH (2017) A randomized controlled clinical trial comparing small buccal dehiscence defects around dental implants treated with guided bone regeneration or left for spontaneous healing. Clin Oral Implants Res 28:348–354. https://doi.org/10.1111/clr.12806
Monje A, Chappuis V, Monje F, Munoz F, Wang HL, Urban IA, Buser D (2019) The critical peri-implant buccal bone wall thickness revisited: an experimental study in the beagle dog. Int J Oral Maxillofac Implants 34:1328–1336. https://doi.org/10.11607/jomi.7657
Acknowledgments
The authors would like to express their appreciation to the personnel of the Rof Codina research facilities, for their invaluable support with the care of the animals. Also, the authors would like to acknowledge the support with the histological processing made by Fernando Muñoz’s research team.
Funding
This study was partially funded through a research contract between the University Complutense of Madrid and MIS Implants (Israel).
Author information
Authors and Affiliations
Contributions
• Javier Sanz-Esporrin: data retrieval, data analysis, help in surgical procedures, and writing the manuscript.
• Riccardo Di Raimondo: helped in surgeries in dogs.
• Rafael Pla: helped in surgeries in dogs.
• Fernando Luengo: helped in surgeries in dogs.
• Fabio Vignoletti: surgeries in dogs, protocol design, and manuscript editing.
• Javier Núñez: surgeries in dogs and manuscript editing.
• Georgios Antonoglou: histomorphometry measurements and draft preparation.
• Juan Blanco: surgeries in dogs, protocol design, and manuscript editing.
• Mariano Sanz: protocol design and manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 219 kb)
Rights and permissions
About this article
Cite this article
Sanz-Esporrin, J., Di Raimondo, R., Pla, R. et al. Experimental peri-implantitis around titanium implants with a chemically modified surface with a monolayer of multi-phosphonate molecules: a preclinical in vivo investigation. Clin Oral Invest 25, 3789–3800 (2021). https://doi.org/10.1007/s00784-020-03708-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03708-4