Abstract
Objectives
Biphasic calcium phosphate (BCP) is a bioceramic material successfully used in alloplastic bone augmentation. Despite many advantages, a disadvantage of BCP seems to be a difficult application and position instability. The aim of this study was to determine how different carrier materials influence BCP-induced quantitative and qualitative bone regeneration.
Materials and methods
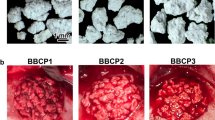
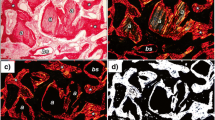
A total of 70 critical size defects were set in the frontal bone of 14 domestic pigs (5 each) and filled randomly with either BCP alone (BCP), BCP in combination with nano-hydroxyapatite (BCP + NHA), BCP embedded in native porcine type I/III collagen blocks (BCP + C), autologous bone (AB), or were left empty (ED). Specimens were harvested after 4 and 8 weeks and were evaluated histologically as well as histomorphometrically.
Results
Significantly lowest rate of new bone formation was found in ED (p = < 0.001) and BCP + NHA groups (p = 0.05). After 8 weeks, the highest percentage of new bone formation was observed in the BCP + C group. Fibrous matrix was detected highest in BCP alone. The lowest residual bone substitute material was found in BCP + C after 8 weeks.
Conclusions
BCP-induced bone regeneration is indeed affected by different carrier types. Surface morphology and bioactive characteristics influence osseointegration and new bone formation in vivo. The combination of type I/III collagen seems most suitable for qualitative and quantitative bone regeneration.
Clinical relevance
Stabilization of granular bone substitutes using type I/III collagen might be an alternative to granulates alone, indicating excellent volume stability, satisfactory plasticity, and easy application.




Similar content being viewed by others
References
Clementini M, Morlupi A, Canullo L, Agrestini C, Barlattani A (2012) Success rate of dental implants inserted in horizontal and vertical guided bone regenerated areas: a systematic review. Int J Oral Maxillofac Surg 41:847–852
Zouhary KJ (2010) Bone graft harvesting from distant sites: concepts and techniques. Oral Maxillofac Surg Clin N Am 22:301–316. https://doi.org/10.1016/j.coms.2010.04.007
Schorn L, Sproll C, Ommerborn M, Naujoks C, Kubler NR, Depprich R (2017) Vertical bone regeneration using rhBMP-2 and VEGF. Head Face Med 13:11. https://doi.org/10.1186/s13005-017-0146-0
Hulbert S et al (1987) In: Vincenzini P (ed) High tech ceramics
Hench LL (1991) Bioceramics: from concept to clinic. J Am Ceram Soc 74. https://doi.org/10.1111/j.1151-2916.1991.tb07132.x
Jeong J, Kim JH, Shim JH, Hwang NS, Heo CY (2019) Bioactive calcium phosphate materials and applications in bone regeneration. Biomat Res 23:4. https://doi.org/10.1186/s40824-018-0149-3
Kanazawa T, Umegaki T, Monma H (1975) Apatites, new inorganic materials. Ceramics Japan 10
Frank O (2002) Real-time quantitative RT-PCR analysis of human bone marrow stromal cells during osteogenic differentiation in vitro. J Cell Biochem 85. https://doi.org/10.1002/jcb.10174
Orimo H (2010) The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J Nippon Med Sch 77. https://doi.org/10.1272/jnms.77.4
Shea JE, Miller SC (2005) Skeletal function and structure: implications for tissue-targeted therapeutics. Adv Drug Deliv Rev 57. https://doi.org/10.1016/j.addr.2004.12.017
Whited BM (2006) Osteoblast response to zirconia-hybridized pyrophosphate-stabilized amorphous calcium phosphate. J Biomed Mater Res A 76. https://doi.org/10.1002/jbm.a.30573
Liu D (2008) Activation of extracellular-signal regulated kinase (ERK1/2) by fluid shear is Ca2 + −and ATP-dependent in MC3T3-E1 osteoblasts. Bone 42. https://doi.org/10.1016/j.bone.2007.09.058
Danciu TE (2003) Calcium regulates the PI3K-Akt pathway in stretched osteoblasts. FEBS Lett 536. https://doi.org/10.1016/S0014-5793(03)00055-3
Khoshniat S (2011) The emergence of phosphate as a specific signaling molecule in bone and other cell types in mammals. Cell Mol Life Sci 68. https://doi.org/10.1007/s00018-010-0527-z
Julien M (2009) Phosphate-dependent regulation of MGP in osteoblasts: role of ERK1/2 and Fra-1. J Bone Miner Res 24. https://doi.org/10.1359/jbmr.090508
Mozar A (2008) High extracellular inorganic phosphate concentration inhibits RANK–RANKL signaling in osteoclast-like cells. J Cell Physiol 215. https://doi.org/10.1002/jcp.21283
Ambard AJ, Mueninghoff L (2006) Calcium phosphate cement: review of mechanical and biological properties. J Prosthodont 15. https://doi.org/10.1111/j.1532-849X.2006.00129.x.
Samavedi S, Whittington AR, Goldstein AS (2013) Calcium phosphate ceramics in bone tissue engineering: a review of properties and their influence on cell behavior. Acta Biomater 9. https://doi.org/10.1016/j.actbio.2013.06.014
Arinzeh TL (2005) A comparative study of biphasic calcium phosphate ceramics for human mesenchymal stem-cell-induced bone formation. Biomaterials 26. https://doi.org/10.1016/j.biomaterials.2004.09.035
He F (2016) Comparative study on in vivo response of porous calcium carbonate composite ceramic and biphasic calcium phosphate ceramic. Mater Sci Eng C 64. https://doi.org/10.1016/j.msec.2016.03.085
Le Nihouannen D, Duval L, Lecomte A, Julien M, Guicheux J, Daculsi G, Layrolle P (2007) Interactions of total bone marrow cells with increasing quantities of macroporous calcium phosphate ceramic granules. J Mater Sci Mater Med 18:1983–1990. https://doi.org/10.1007/s10856-007-3098-2
Schwartz C, Liss P, Jacquemaire B, Lecestre P, Frayssinet P (1999) Biphasic synthetic bone substitute use in orthopaedic and trauma surgery: clinical, radiological and histological results. J Mater Sci Mater Med 10:821–825
Schmitz JP, Hollinger JO (1986) The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res:299–308
Fienitz T, Moses O, Klemm C, Happe A, Ferrari D, Kreppel M, Ormianer Z, Gal M, Rothamel D (2016) Histological and radiological evaluation of sintered and non-sintered deproteinized bovine bone substitute materials in sinus augmentation procedures. A prospective, randomized-controlled, clinical multicenter study. Clin Oral Investig. https://doi.org/10.1007/s00784-016-1829-9
Fienitz T, Moses O, Klemm C, Happe A, Ferrari D, Kreppel M, Ormianer Z, Gal M, Rothamel D (2017) Histological and radiological evaluation of sintered and non-sintered deproteinized bovine bone substitute materials in sinus augmentation procedures. A prospective, randomized-controlled, clinical multicenter study. Clin Oral Investig 21:787–794. https://doi.org/10.1007/s00784-016-1829-9
Rothamel D, Schwarz F, Fienitz T, Smeets R, Dreiseidler T, Ritter L, Happe A, Zoller J (2012) Biocompatibility and biodegradation of a native porcine pericardium membrane: results of in vitro and in vivo examinations. Int J Oral Maxillofac Implants 27:146–154
Donath K, Breuner G (1982) A method for the study of undecalcified bones and teeth with attached soft tissues. The Sage-Schliff (sawing and grinding) technique. J Oral Pathol 11:318–326
Bighetti ACC, Cestari TM, Santos PS, Arantes RVN, Paini S, Assis GF, Costa BC, de Oliveira FA, Tokuhara CK, de Oliveira RC, Taga R (2020) In vitro and in vivo assessment of CaP materials for bone regenerative therapy. The role of multinucleated giant cells/osteoclasts in bone regeneration. J Biomed Mater Res B Appl Biomater 108:282–297. https://doi.org/10.1002/jbm.b.34388.
Klein M, Al-Nawas B (2011) For which clincal indications in dental implantology is the use of bone substitute materials scientifically substantiated? Eur J Oral Implantol 4:11–29
Fahmy RA, Mahmoud N, Soliman S, Nouh SR, Cunningham L, El-Ghannam A (2015) Acceleration of alveolar ridge augmentation using a low dose of recombinant human bone morphogenetic protein-2 loaded on a resorbable bioactive ceramic. J Oral Maxillofac Surg 73:2257–2272. https://doi.org/10.1016/j.joms.2015.07.004
Felice P, Marchetti C, Piattelli A, Pellegrino G, Checchi V, Worthington H, Esposito M (2008) Vertical ridge augmentation of the atrophic posterior mandible with interpositional block grafts: bone from the iliac crest versus bovine anorganic bone. Eur J Oral Implantol 1:183–198
Troeltzsch M, Troeltzsch M, Kauffmann P, Gruber R, Brockmeyer P, Moser N, Rau A, Schliephake H (2016) Clinical efficacy of grafting materials in alveolar ridge augmentation: A systematic review. J Cranio-maxillo-facial Surg 44:1618–1629. https://doi.org/10.1016/j.jcms.2016.07.028
Rothamel D, Neugebauer J, Lingohr T, Dreiseidler T, Ritter L, Zöller J (2009) Oberflächenstruktur, Biokompatibilität und Hartgewebsregeneration. Z Oral Implant:2–9
Merten HA, Wiltfang J, Grohmann U, Hoenig JF (2001) Intraindividual comparative animal study of alpha- and beta-tricalcium phosphate degradation in conjunction with simultaneous insertion of dental implants. J Craniofac Surg 12:59–68
Fujita R, Yokoyama A, Kawasaki T, Kohgo T (2003) Bone augmentation osteogenesis using hydroxyapatite and beta-tricalcium phosphate blocks. J Oral Maxillofac Surg 61:1045–1053
LeGeros RZ, Lin S, Rohanizadeh R, Mijares D, LeGeros JP (2003) Biphasic calcium phosphate bioceramics: preparation, properties and applications. J Mat Sci Mat Med 14:201–209
Jensen SS, Yeo A, Dard M, Hunziker E, Schenk R, Buser D (2007) Evaluation of a novel biphasic calcium phosphate in standardized bone defects: a histologic and histomorphometric study in the mandibles of minipigs. Clin Oral Implants Res 18:752–760. https://doi.org/10.1111/j.1600-0501.2007.01417.x
Giesenhagen B (2008) Die einzeitige vertikale Augmentation mit ringförmigen Knochentransplantaten. Z Zahnärztl Impl 24:129–132
Carrel JP, Wiskott A, Moussa M, Rieder P, Scherrer S, Durual S (2016) A 3D printed TCP/HA structure as a new osteoconductive scaffold for vertical bone augmentation. Clin Oral Implants Res 27:55–62. https://doi.org/10.1111/clr.12503
Cardaropoli G, Araujo M, Hayacibara R, Sukekava F, Lindhe J (2005) Healing of extraction sockets and surgically produced - augmented and non-augmented - defects in the alveolar ridge. An experimental study in the dog. J Clin Periodontol 32:435–440. https://doi.org/10.1111/j.1600-051X.2005.00692.x
Meyer U, Meyer T, Handschel J, Wiesmann HP (2009) Fundamentals of Tissue Engineering and Regenerative Medicine. Springer-Verlag, Heidelberg
Mygind T (2007) Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials 28. https://doi.org/10.1016/j.biomaterials.2006.10.003
Webster TJ et al (2000) Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J Biomed Mater Res: an official journal of the Society for Biomaterials, the Japanese Society for Biomaterials, and the Australian Society for Biomaterials and the Korean society for. Biomaterials 51:475–483
Deligianni DD (2000) Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 22. https://doi.org/10.1016/S0142-9612(00)00174-5
Fellah BH, Gauthier O, Weiss P, Chappard D, Layrolle P (2008) Osteogenicity of biphasic calcium phosphate ceramics and bone autograft in a goat model. Biomaterials 29:1177–1188. https://doi.org/10.1016/j.biomaterials.2007.11.034
Chapman MW, Bucholz R, Cornell C (1997) Treatment of acute fractures with a collagen-calcium phosphate graft material. A randomized clinical trial. J Bone Joint Surg Am 79:495–502
Thorwarth M, Schultze-Mosgau S, Kessler P, Wiltfang J, Schlegel KA (2005) Bone regeneration in osseous defects using a resorbable nanoparticular hydroxyapatite. J Oral Maxillofac Surg 63:1626–1633. https://doi.org/10.1016/j.joms.2005.06.010
Hu J, Zhou Y, Huang L, Liu J, Lu H (2014) Effect of nano-hydroxyapatite coating on the osteoinductivity of porous biphasic calcium phosphate ceramics. BMC Musculoskelet Disord 15:114. https://doi.org/10.1186/1471-2474-15-114
Herten M, Rothamel D, Schwarz F, Friesen K, Koegler G, Becker J (2009) Surface- and nonsurface-dependent in vitro effects of bone substitutes on cell viability. Clin Oral Investig 13:149–155. https://doi.org/10.1007/s00784-008-0214-8
Rothamel D, Schwarz F, Herten M, Berndsen K, Steigmann M, Neugebauer J, Becker J (2008) Vertical augmentation of the mandible using cortico-spongious xenoblocks. A histomorphometrical study in dogs. Schweiz Monatsschr Zahnmed 118:1162–1169
Aloise AC, Pelegrine AA, Zimmermann A, de Mello EOR, Ferreira LM (2015) Repair of critical-size bone defects using bone marrow stem cells or autogenous bone with or without collagen membrane: a histomorphometric study in rabbit calvaria. Int J Oral Maxillofac Implants 30:208–215. https://doi.org/10.11607/jomi.4010
Paknejad M, Rokn AR, Yaghobee S, Moradinejad P, Heidari M, Mehrfard A (2014) Effects of two types of anorganic bovine bone on bone regeneration: a histological and histomorphometric study of rabbit calvaria. J Dentist (Tehran, Iran) 11:687–695
Hermansen K, Pedersen LE, Olesen HO (1986) The analgesic effect of buprenorphine, etorphine and pethidine in the pig: a randomized double blind cross-over study. Acta Pharmacol Toxicol (Copenh) 59:27–35. https://doi.org/10.1111/j.1600-0773.1986.tb00130.x.
Papich MG (2008) An update on nonsteroidal anti-inflammatory drugs (NSAIDs) in small animals. Vet Clin North Am Small Anim Pract 38(1243-1266):vi. https://doi.org/10.1016/j.cvsm.2008.09.002
Boshra V (2011) Evaluation of osteoporosis risk associated with chronic use of morphine, fentanyl and tramadol in adult female rats. Curr Drug Saf 6:159–163. https://doi.org/10.2174/157488611797579267
Coluzzi F, Pergolizzi J, Raffa RB, Mattia C (2015) The unsolved case of “bone-impairing analgesics”: the endocrine effects of opioids on bone metabolism. Ther Clin Risk Manag 11:515–523. https://doi.org/10.2147/TCRM.S79409.
Xu H, Shimizu Y, Asai S, Ooya K (2003) Experimental sinus grafting with the use of deproteinized bone particles of different sizes. Clin Oral Implants Res 14:548–555
Funding
The study was financially supported by Bego Implant Systems, Bremen, Germany.
Author information
Authors and Affiliations
Contributions
L.S. wrote the manuscript; T.F., M.G, A. S-K., and A. M. supervised and conducted the experiments and collected the data; J.L. and H.H. revised the manuscript critically; R. D. initiated the research and had the idea to publish this paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors claim that Prof. Daniel Rothamel is a founder and shareholder of Botiss Biomaterials, Berlin, Germany, the manufacturer of biomaterials used in this study.
Ethical statements
This study has been approved by the state office for nature, environment, and consumer protection of North-Rhine Westphalia, Germany (LANUV NRW AZ 84-02.04.2011.A148). It complies with the ARRIVE guidelines for animal research.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 96 kb)
Rights and permissions
About this article
Cite this article
Schorn, L., Fienitz, T., Gerstenberg, M.F. et al. Influence of different carrier materials on biphasic calcium phosphate induced bone regeneration. Clin Oral Invest 25, 3729–3737 (2021). https://doi.org/10.1007/s00784-020-03700-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03700-y