Abstract
Objectives
This study aims to evaluate the cytocompatibility of three provisional restoration materials and predict neurotoxic potential of their monomers. These materials are Tab 2000® (methyl methacrylate based), ProTemp 4™ (bis-acrylic based) and Structur 3® (urethane dimethacrylate based).
Materials and methods
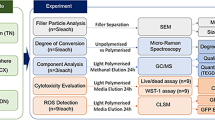
Resin samples were incubated in a cell culture medium and the cytotoxic effects of these extracts were studied in 3T3 fibroblast cells through MTT and crystal violet assays as well as ROS assessment. The presence of relevant leached monomers was determined by HPLC. Additionally, the blood-brain barrier (BBB) permeability to these resin-based monomers was predicted using ACD/Labs algorithms model.
Results
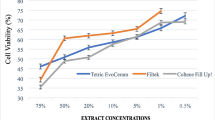
Cell survival rates were compared with the resin extracts, and Structur 3® was statistically significant different from the others (p < 0.001) at all-time incubation periods. All materials induced a dose-dependent loss of cell viability; however, only Structur 3 extracts were cytotoxic against 3T3 fibroblasts. The highest cytotoxic effect (77%, p < 0.001) was observed at 24 h incubation period, which may be associated with the presence of urethane dimethacrylate (UDMA) leached monomers. Furthermore, the computational model showed that most monomers under study are expectedly capable of crossing the BBB.
Conclusions
Our results showed that Structur 3® is not cytocompatible with our cell model and UDMA is a potential neurotoxic compound.
Clinical relevance
These results indicate that only ProTemp 4™ and Tab 2000® are safe for provisional restorations.






Similar content being viewed by others
References
Rakhshan V (2015) Marginal integrity of provisional resin restoration materials: a review of the literature. Saudi J Dent Res 6:33–40. https://doi.org/10.1016/j.sjdr.2014.03.002
Burns DR, Beck DA, Nelson SK (2003) A review of selected dental literature on contemporary provisional fixed prosthodontic treatment: report of the Committee on Research in Fixed Prosthodontics of the Academy of Fixed Prosthodontics. J Prosthet Dent 90:474–497. https://doi.org/10.1016/S0022-3913(03)00259-2
Ferracane JL (2011) Resin composite - state of the art. Dent Mater 27:29–38. https://doi.org/10.1016/j.dental.2010.10.020
Zabrovsky A, Beyth N, Pietrokovski Y, et al (2017) 5 – Biocompatibility and functionality of dental restorative materials. In: Shelton R (ed) Biocompatibility of dental biomaterials. Woodhead Publishing Series in Biomaterials, pp 63–75
Gautam R, Singh RD, Sharma VP et al (2012) Biocompatibility of polymethylmethacrylate resins used in dentistry. J Biomed Mater Res Part B Appl Biomater 100B:1444–1450. https://doi.org/10.1002/jbm.b.32673
Wataha JC (2012) Predicting clinical biological responses to dental materials. Dent Mater 28:23–40. https://doi.org/10.1016/j.dental.2011.08.595
Schmalz G, Galler KM (2017) Biocompatibility of biomaterials – lessons learned and considerations for the design of novel materials. Dent Mater 33:382–393. https://doi.org/10.1016/j.dental.2017.01.011
Luthardt RG, Stößel M, Hinz M, Vollandt R (2000) Clinical performance and periodontal outcome of temporary crowns and fixed partial dentures: a randomized clinical trial. J Prosthet Dent 83:32–39. https://doi.org/10.1016/S0022-3913(00)70086-2
Van Landuyt KL, Nawrot T, Geebelen B et al (2011) How much do resin-based dental materials release? A meta-analytical approach. Dent Mater 27:723–747. https://doi.org/10.1016/j.dental.2011.05.001
Sideridou ID, Achilias DS (2005) Elution study of unreacted Bis-GMA, TEGDMA, UDMA, and Bis-EMA from light-cured dental resins and resin composites using HPLC. J Biomed Mater Res Part B Appl Biomater 74B:617–626. https://doi.org/10.1002/jbm.b.30252
Labban N, Song F, Al-Shibani N, Windsor LJ (2008) Effects of provisional acrylic resins on gingival fibroblast cytokine/growth factor expression. J Prosthet Dent 100:390–397. https://doi.org/10.1016/S0022-3913(08)60242-5
Borzangy S, Labban N, Windsor LJ (2013) Effects of interim acrylic resins on the expression of cytokines from epithelial cells and on collagen degradation. J Prosthet Dent 110:296–302. https://doi.org/10.1016/S0022-3913(13)60379-0
Donaldson D (1974) The etiology of gingival recession associated with temporary crowns. J Periodontol 45:468–471. https://doi.org/10.1902/jop.1974.45.7.468
Sigusch BW, Pflaum T, Völpel A, Gretsch K, Hoy S, Watts DC, Jandt KD (2012) Resin-composite cytotoxicity varies with shade and irradiance. Dent Mater 28:312–319. https://doi.org/10.1016/j.dental.2011.12.007
Ulker M, Ulker HE, Zortuk M, Bulbul M, Tuncdemir AR, Bilgin MS (2009) Effects of current provisional restoration materials on the viability of fibroblasts. Eur J Dent 3:114–119
Ergün G, Mutlu-Sagesen L, Karaoglu T, Dogan A (2001) Cytotoxicity of provisional crown and bridge restoration materials: an in vitro study. J Oral Sci 43:123–128
Quinlan CA, Zisterer DM, Tipton KF, O’Sullivan MI (2002) In vitro cytotoxicity of a composite resin and compomer. Int Endod J 35:47–55. https://doi.org/10.1046/j.1365-2591.2002.00456.x
ISO 10993-5 (2009) Biological evaluation of medical devices - part 5: tests for in vitro cytotoxicity. International Organization for Standardization, Geneva
ISO 7405 (2018) Dentistry - Evaluation of biocompatibility of medical devices used in dentistry. International Organization for Standardization, Geneva
Leggat PA, Kedjarune U (2003) Toxicity of methyl methacrylate in dentistry. Int Dent J 53:126–131. https://doi.org/10.1111/j.1875-595X.2003.tb00736.x
Rajaniemi R (1986) Clinical evaluation of occupational toxicity of methylmethacrylate monomer to dental technicians. J Soc Occup Med 36:56–59. https://doi.org/10.1093/occmed/36.4.56
Cornhill JF, Levesque MJ, Herderick EE et al (1980) Quantitative study of the rabbit aortic endothelium using vascular casts. Atherosclerosis 35:321–337. https://doi.org/10.1016/0021-9150(80)90130-6
Sugaya Y, Sakamoto N, Ohashi T, Sato M (2003) Elongation and random orientation of bovine endothelial cells in response to hydrostatic pressure: comparison with response to shear stress. JSME Int J Ser C 46:1248–1255. https://doi.org/10.1299/jsmec.46.1248
Gul P, Miloglu FD, Akgul N (2014) HPLC Analysis of eluted monomers from dental composite using different immersion media. J Liq Chromatogr Relat Technol 37:155–170. https://doi.org/10.1080/10826076.2012.738619
Lanevskij K, Dapkunas J, Juska L, Japertas P, Didziapetris R (2011) QSAR analysis of blood–brain distribution: the influence of plasma and brain tissue binding. J Pharm Sci 100:2147–2160. https://doi.org/10.1002/jps.22442
Lanevskij K, Japertas P, Didziapetris R, Petrauskas A (2009) Ionization-specific QSAR models of blood-brain penetration of drugs. Chem Biodivers 6:2050–2054. https://doi.org/10.1002/cbdv.200900079
Ritz C, Streibig JC (2007) Bioassay analysis using R. J Stat Softw 12:1–22. https://doi.org/10.18637/jss.v012.i05
Bettencourt AF, Neves CB, de Almeida MS, Pinheiro LM, Oliveira SA, Lopes LP, Castro MF (2010) Biodegradation of acrylic based resins: a review. Dent Mater 26:e171–e180. https://doi.org/10.1016/j.dental.2010.01.006
Bakopoulou A, Papadopoulos T, Garefis P (2009) Molecular toxicology of substances released from resin–based dental restorative materials. Int J Mol Sci 10:3861–3899. https://doi.org/10.3390/ijms10093861
Goldberg M (2008) In vitro and in vivo studies on the toxicity of dental resin components: a review. Clin Oral Investig 12:1–8. https://doi.org/10.1007/s00784-007-0162-8
Gupta SK, Saxena P, Pant VA, Pant AB (2012) Release and toxicity of dental resin composite. Toxicol Int 19:225–234. https://doi.org/10.4103/0971-6580.103652
Volk J, Engelmann J, Leyhausen G, Geurtsen W (2006) Effects of three resin monomers on the cellular glutathione concentration of cultured human gingival fibroblasts. Dent Mater 22:499–505. https://doi.org/10.1016/j.dental.2005.06.002
Reichl F-X, Esters M, Simon S, Seiss M, Kehe K, Kleinsasser N, Folwaczny M, Glas J, Hickel R (2006) Cell death effects of resin-based dental material compounds and mercurials in human gingival fibroblasts. Arch Toxicol 80:370–377. https://doi.org/10.1007/s00204-005-0044-2
Engelmann J, Janke V, Volk J, Leyhausen G, von Neuhoff N, Schlegelberger B, Geurtsen W (2004) Effects of BisGMA on glutathione metabolism and apoptosis in human gingival fibroblasts in vitro. Biomaterials 25:4573–4580. https://doi.org/10.1016/j.biomaterials.2003.11.048
Chang H-H, Chang M-C, Wang H-H, Huang GF, Lee YL, Wang YL, Chan CP, Yeung SY, Tseng SK, Jeng JH (2014) Urethane dimethacrylate induces cytotoxicity and regulates cyclooxygenase-2, hemeoxygenase and carboxylesterase expression in human dental pulp cells. Acta Biomater 10:722–731. https://doi.org/10.1016/j.actbio.2013.10.006
Kleinsasser NH, Schmid K, Sassen AW, Harréus UA, Staudenmaier R, Folwaczny M, Glas J, Reichl FX (2006) Cytotoxic and genotoxic effects of resin monomers in human salivary gland tissue and lymphocytes as assessed by the single cell microgel electrophoresis (Comet) assay. Biomaterials 27:1762–1770. https://doi.org/10.1016/j.biomaterials.2005.09.023
Schweikl H, Schmalz G, Spruss T (2001) The induction of micronuclei in vitro by unpolymerized resin monomers. J Dent Res 80:1615–1620. https://doi.org/10.1177/00220345010800070401
Wisniewska-Jarosinska M, Poplawski T, Chojnacki CJ, Pawlowska E, Krupa R, Szczepanska J, Blasiak J (2011) Independent and combined cytotoxicity and genotoxicity of triethylene glycol dimethacrylate and urethane dimethacrylate. Mol Biol Rep 38:4603–4611. https://doi.org/10.1007/s11033-010-0593-1
Durner J, Wellner P, Hickel R, Reichl FX (2012) Synergistic interaction caused to human gingival fibroblasts from dental monomers. Dent Mater 28:818–823. https://doi.org/10.1016/j.dental.2012.04.031
Siqueira Gonçalves T, Minghelli Schmitt V, Thomas M, Lopes de Souza MA, Macedo de Menezes L (2008) Cytotoxicity of two autopolymerized acrylic resins used in orthodontics. Angle Orthod 78:926–930. https://doi.org/10.2319/072407-343.1
Pupo YM, Bernardo CF, de Souza FFFA et al (2017) Cytotoxicity of etch-and-rinse, self-etch, and universal dental adhesive systems in fibroblast cell line 3T3. Scanning 2017:1–7. https://doi.org/10.1155/2017/9650420
Olivier A, Grobler SR, Osman Y (2012) Cytotoxicity of seven recent dentine bonding agents on mouse 3T3 fibroblast cells. Open J Stomatol 02:244–250. https://doi.org/10.4236/ojst.2012.24043
Kurt A, Altintas SH, Kiziltas MV, Tekkeli SE, Guler EM, Kocyigit A, Usumez A (2018) Evaluation of residual monomer release and toxicity of self-adhesive resin cements. Dent Mater J 37:40–48. https://doi.org/10.4012/dmj.2016-380
Diomede F, Caputi S, Merciaro I, Frisone S, D'Arcangelo C, Piattelli A, Trubiani O (2014) Pro-inflammatory cytokine release and cell growth inhibition in primary human oral cells after exposure to endodontic sealer. Int Endod J 47:864–872. https://doi.org/10.1111/iej.12230
Yoshii E (1997) Cytotoxic effects of acrylates and methacrylates: relationships of monomer structures and cytotoxicity. J Biomed Mater Res 37:517–524. https://doi.org/10.1002/(SICI)1097-4636(19971215)37:4<517::AID-JBM10>3.0.CO;2-5
Geurtsen W, Lehmann F, Spahl W, Leyhausen G (1998) Cytotoxicity of 35 dental resin composite monomers/additives in permanent 3T3 and three human primary fibroblast cultures. J Biomed Mater Res 41:474–480. https://doi.org/10.1002/(SICI)1097-4636(19980905)41:3<474::AID-JBM18>3.0.CO;2-I
Walters NJ, Xia W, Salih V, Ashley PF, Young AM (2016) Poly(propylene glycol) and urethane dimethacrylates improve conversion of dental composites and reveal complexity of cytocompatibility testing. Dent Mater 32:264–277. https://doi.org/10.1016/j.dental.2015.11.017
Rose EC, Bumann J, Jonas IE, Kappert HF (2000) Contribution to the biological assessment of orthodontic acrylic materials. Measurement of their residual monomer output and cytotoxicity. J Orofac Orthop / Fortschritte der Kieferorthopädie 61:246–257. https://doi.org/10.1007/s000560050010
Schulz SD, Laquai T, Kümmerer K et al (2015) Elution of monomers from provisional composite materials. Int J Polym Sci 2015:1–7. https://doi.org/10.1155/2015/617407
Moharamzadeh K, Van Noort R, Brook IM, Scutt AM (2007) HPLC analysis of components released from dental composites with different resin compositions using different extraction media. J Mater Sci Mater Med 18:133–137. https://doi.org/10.1007/s10856-006-0671-z
Ferracane JL (1994) Elution of leachable components from composites. J Oral Rehabil 21:441–452. https://doi.org/10.1111/j.1365-2842.1994.tb01158.x
Altintas SH, Usumez A (2008) Evaluation of monomer leaching from a dual cured resin cement. J Biomed Mater Res Part B Appl Biomater 86B:523–529. https://doi.org/10.1002/jbm.b.31052
Bouillaguet S, Shaw L, Gonzalez L, Wataha JC, Krejci I (2002) Long-term cytotoxicity of resin-based dental restorative materials. J Oral Rehabil 29:7–13. https://doi.org/10.1046/j.1365-2842.2002.00804.x
Mohsen NM, Craig RG, Hanks CT (1998) Cytotoxicity of urethane dimethacrylate composites before and after aging and leaching. J Biomed Mater Res 39:252–260. https://doi.org/10.1002/(SICI)1097-4636(199802)39:2<252::AID-JBM12>3.0.CO;2-F
Lefebvre CA, Knoernschild KL, Schuster GS (1994) Cytotoxicity of eluates from light-polymerized denture base resins. J Prosthet Dent 72:644–650. https://doi.org/10.1016/0022-3913(94)90298-4
Acknowledgements
The authors wish to thank Teresa Calejo for the critical revision of the manuscript. The authors are also grateful to Mário Diniz for making the fluorescence microplate reader available for ROS assessment.
Funding
This work was partially supported by FCT—Fundação para a Ciência e a Tecnologia projects—PTDC/SAU-BMA/122444/2010 and PTDC/BIM-MEC/6631/2014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article this not contain any studies with human participants or animals perform by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bandarra, S., Mascarenhas, P., Luís, A.R. et al. In vitro and in silico evaluations of resin-based dental restorative material toxicity. Clin Oral Invest 24, 2691–2700 (2020). https://doi.org/10.1007/s00784-019-03131-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-03131-4