Abstract
Objectives
Craniofacial sutures are important growth sites for skull development and are sensitive to mechanical stress. In order to determine the role of bone resorption in stress-mediated sutural bone growth, midpalatal suture expansion was performed in mice receiving alendronate, an anti-resorptive bisphosphonate.
Materials and methods
The midpalatal sutures of 8-week-old C57BL/6 mice were expanded by orthodontic wires over the period of 2 weeks. Mice with maxillary expansion without drug treatment as well as untreated animals served as controls. Skulls were analyzed with micro-computed tomography (micro-CT), immunohistochemistry and histology.
Results
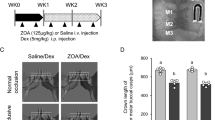
Maxillary expansion in mice without drug treatment resulted in an increase of TRAP-positive osteoclasts. In contrast, no increase in osteoclasts was observed in expanded sutures of mice with bisphosphonate treatment. Double calcein labeling demonstrated rapid bone formation on the oral edges of the expanded sutures in mice without bisphosphonate treatment. Less bone formation was observed in bisphosphonate-treated mice after expansion. Histology revealed that the sutural architecture was reestablished in expanded sutures of mice without bisphosphonate treatment. In contrast, the sutural architecture was disorganized and the cartilage had an irregular form, following expansion in bisphosphonate-treated mice. Finally, micro-CT imaging demonstrated that the total amount of maxillary expansion was significantly lower in mice with bisphosphonate treatment as compared to those of mice without drug treatment.
Conclusions
In conclusion, our results indicate that osteoclast-mediated bone resorption is needed for maxillary suture expansion and reorganization of sutural architecture.
Clinical significance
Orthodontic palatal expansion can be complicated in patients with inherited or drug-induced diseases of osteoclast dysfunction.




Similar content being viewed by others
References
Mao JJ, Nah HD (2004) Growth and development: hereditary and mechanical modulations. Am J Orthod Dentofac Orthop 125(6):676–689. https://doi.org/10.1016/S0889540604001908
Lee K, Sugiyama H, Imoto S, Tanne K (2001) Effects of bisphosphonate on the remodeling of rat sagittal suture after rapid expansion. Angle Orthod 71(4):265–273. https://doi.org/10.1043/0003-3219(2001)071<0265:EOBOTR>2.0.CO;2
Murray JM, Cleall JF (1971) Early tissue response to rapid maxillary expansion in the midpalatal suture of the rhesus monkey. J Dent Res 50(6):1654–1660. https://doi.org/10.1177/00220345710500065101
Uysal T, Ustdal A, Sonmez MF, Ozturk F (2009) Stimulation of bone formation by dietary boron in an orthopedically expanded suture in rabbits. Angle Orthod 79(5):984–990. https://doi.org/10.2319/112708-604.1
Takahashi I, Mizoguchi I, Nakamura M, Sasano Y, Saitoh S, Kagayama M, Mitani H (1996) Effects of expansive force on the differentiation of midpalatal suture cartilage in rats. Bone 18(4):341–348
Kobayashi ET, Hashimoto F, Kobayashi Y, Sakai E, Miyazaki Y, Kamiya T, Kobayashi K, Kato Y, Sakai H (1999) Force-induced rapid changes in cell fate at midpalatal suture cartilage of growing rats. J Dent Res 78(9):1495–1504. https://doi.org/10.1177/00220345990780090301
Wang H, Sun W, Ma J, Pan Y, Wang L, Zhang WB (2015) Biglycan mediates suture expansion osteogenesis via potentiation of Wnt/beta-catenin signaling. J Biomech 48(3):432–440. https://doi.org/10.1016/j.jbiomech.2014.12.032
Hou B, Kolpakova-Hart E, Fukai N, Wu K, Olsen BR (2009) The polycystic kidney disease 1 (Pkd1) gene is required for the responses of osteochondroprogenitor cells to midpalatal suture expansion in mice. Bone 44(6):1121–1133. https://doi.org/10.1016/j.bone.2009.02.018
Hou B, Fukai N, Olsen BR (2007) Mechanical force-induced midpalatal suture remodeling in mice. Bone 40(6):1483–1493. https://doi.org/10.1016/j.bone.2007.01.019
Bertola D, Amaral C, Kim C, Albano L, Aguena M, Passos-Bueno MR (2010) Craniosynostosis in pycnodysostosis: broadening the spectrum of the cranial flat bone abnormalities. Am J Med Genet A 152A(10):2599–2603. https://doi.org/10.1002/ajmg.a.33609
Kawata T, Tokimasa C, Fujita T, Kawasoko S, Kaku M, Sugiyama H, Tanne K (1998) Midpalatal suture of osteopetrotic (op/op) mice exhibits immature fusion. Exp Anim 47(4):277–281
Altan BA, Kara IM, Nalcaci R, Ozan F, Erdogan SM, Ozkut MM, Inan S (2013) Systemic propolis stimulates new bone formation at the expanded suture: a histomorphometric study. Angle Orthod 83(2):286–291. https://doi.org/10.2319/032612-253.1
Fisher JE, Rogers MJ, Halasy JM, Luckman SP, Hughes DE, Masarachia PJ, Wesolowski G, Russell RG, Rodan GA, Reszka AA (1999) Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc Natl Acad Sci U S A 96(1):133–138
Ozturk F, Babacan H, Inan S, Gumus C (2011) Effects of bisphosphonates on sutural bone formation and relapse: a histologic and immunohistochemical study. Am J Orthod Dentofac Orthop 140(1):e31–e41. https://doi.org/10.1016/j.ajodo.2010.11.020
Hayami T, Pickarski M, Wesolowski GA, McLane J, Bone A, Destefano J, Rodan GA, Duong LT (2004) The role of subchondral bone remodeling in osteoarthritis: reduction of cartilage degeneration and prevention of osteophyte formation by alendronate in the rat anterior cruciate ligament transection model. Arthritis Rheum 50(4):1193–1206. https://doi.org/10.1002/art.20124
Chen X, Wang Z, Duan N, Zhu G, Schwarz EM, Xie C (2017) Osteoblast-osteoclast interactions. Connect Tissue Res:1–9. https://doi.org/10.1080/03008207.2017.1290085
Karras JC, Miller JR, Hodges JS, Beyer JP, Larson BE (2009) Effect of alendronate on orthodontic tooth movement in rats. Am J Orthod Dentofac Orthop 136(6):843–847. https://doi.org/10.1016/j.ajodo.2007.11.035
Woo SB, Hande K, Richardson PG (2005) Osteonecrosis of the jaw and bisphosphonates. N Engl J Med 353(1):99–102 discussion 199-102
Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R (1998) Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med 339(14):947–952. https://doi.org/10.1056/NEJM199810013391402
Jurimae J (2010) Interpretation and application of bone turnover markers in children and adolescents. Curr Opin Pediatr 22(4):494–500. https://doi.org/10.1097/MOP.0b013e32833b0b9e
Holberg C, Steinhauser S, Rudzki-Janson I (2007) Rapid maxillary expansion in adults: cranial stress reduction depending on the extent of surgery. Eur J Orthod 29(1):31–36. https://doi.org/10.1093/ejo/cjl067
Fischer-Brandies H, Es-Souni M, Kock N, Raetzke K, Bock O (2003) Transformation behavior, chemical composition, surface topography and bending properties of five selected 0.016″ × 0.022″ NiTi archwires. J Orofac Orthop 64(2):88–99. https://doi.org/10.1007/s00056-003-0062-8
Jilka RL (2013) The relevance of mouse models for investigating age-related bone loss in humans. J Gerontol A Biol Sci Med Sci 68(10):1209–1217. https://doi.org/10.1093/gerona/glt046
Acknowledgments
We thank Tonia Bargmann for critically reading the manuscript.
Funding
The work was supported by the Department of Orthodontics University Medical Center Hamburg-Eppendorf, Hamburg, Germany, by Institute of Osteology and Biomechanics, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, and by the Department of Orthodontics, Giessen and Marburg University Hospital, Marburg Campus, Marburg, Germany. The work was funded by a grant of the German Orthodontic Society to HKS (grant no. 23).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Koehne, T., Kahl-Nieke, B., Amling, M. et al. Inhibition of bone resorption by bisphosphonates interferes with orthodontically induced midpalatal suture expansion in mice. Clin Oral Invest 22, 2345–2351 (2018). https://doi.org/10.1007/s00784-018-2335-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2335-z