Abstract
Objectives
The aim of the present study was to evaluate cytotoxic effects and cytokine production of calcium silicate-based sealers (EndoSeal, EndoSequence BC Sealer, and MTA Fillapex) using an in vitro root canal filling model and three-dimensional (3D) cell culture. AH Plus as a reference was compared to contemporary calcium silicate cements regarding cell viability and cytokine production.
Material and methods
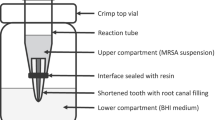
Root canals of 30 human maxillary incisors were prepared using a single-file reciprocating technique. The samples were randomly distributed and canals filled with either AH Plus, EndoSeal, EndoSequence BC Sealer, and MTA Fillapex (n = 6). In the negative control group, the root canal remained unfilled. Sealers were placed into the canals along with a gutta-percha cone placed to working length. Balb/c 3T3 fibroblasts, cultured in a type I collagen 3D scaffold, were exposed to filling material and the respective root apex for 24 h. Cytocompatibility of the materials was evaluated using the methyl-thiazoldiphenyl-tetrazolium (MTT) assay. The production of IL-1β, IL-6, and IL-8 was analyzed using enzyme-linked immunosorbent assay (ELISA). One-way analysis of variance was performed, and when the F-ratios were significant, data were compared by Duncan’s multiple-range test. The alpha-type error was set at 0.05.
Results
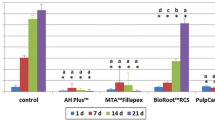
EndoSeal, Endosequence BC Sealer and AH Plus showed cell viability that was similar to the negative control group (P > 0.05), while MTA Fillapex sealer was cytotoxic (P < 0.05). Varying production of IL-1β, IL-6, and IL-8 was detected in all samples.
Conclusions
In an in vitro root canal filling model with 3D cell culture, AH Plus, EndoSeal, and EndoSequence BC Sealer were cytocompatible.
Clinical relevance
These results may suggest that AH Plus, EndoSeal and EndoSequence BC Sealer may achieve better biological response when compared to MTA Fillapex.


Similar content being viewed by others
References
Parirokh M, Torabinejad M (2010a) Mineral trioxide aggregate: a comprehensive literature review—part I: chemical, physical and antibacterial properties. J Endod 36:16–27
Torabinejad M, Parirokh M (2010) Mineral trioxide aggregate: a comprehensive literature review—part II: leakage and biocompatibility investigations. J Endod 36:190–202
Parirokh M, Torabinejad M (2010b) Mineral trioxide aggregate: a comprehensive literature review—part III: clinical applications, drawbacks, and mechanism of action. J Endod 36:400–413
Ricucci D, Langeland K (1998) Apical limit of root canal instrumentation and obturation, part 2. A histological study. Int Endod J 31:394–409
Ricucci D, Rôças IN, Alves FR, Loghin S, Siqueira JF Jr (2016) Apically extruded sealers: fate and influence on treatment outcome. J Endod 42:243–249
Peters OA (2013) Research that matters—biocompatibility and cytotoxicity screening. Int Endod J 46:195–197
Mueller-Klieser W (1997) Three-dimensional cell cultures: from molecular mechanisms to clinical applications. Am J Phys 273:1109–1123
Carletti E, Motta A, Migliaresi C (2011) Scaffolds for tissue engineering and 3D cell culture. Meth Mol Biol 695:17–39
Silva EJ, Senna PM, De-Deus G, Zaia AA (2016b) Cytocompatibility of Biodentine using a three-dimensional cell culture model. Int Endod J 49:574–580
Silva EJ, Rosa TP, Herrera DR, Jacinto RC, Gomes BP, Zaia AA (2013) Evaluation of cytotoxicity and physicochemical properties of calcium silicate-based endodontic sealer MTA Fillapex. J Endod 39:274–277
Zhou HM, TF D, Shen Y, Wang ZJ, Zheng YF, Haapasalo M (2015) In vitro cytotoxicity of calcium silicate-containing endodontic sealers. J Endod 41:56–61
Rodríguez-Lozano FJ, García-Bernal D, Oñate-Sánchez RE, Ortolani-Seltenerich PS, Forner L, Moraleda JM (2016) Evaluation of cytocompatibility of calcium silicate-based endodontic sealers and their effects on the biological responses of mesenchymal dental stem cells. Int Endod J. doi:10.1111/iej.12596
Vitti RP, Prati C, Sinhoreti MA, et al. (2013) Chemical-physical properties of experimental root canal sealers based on butyl ethylene glycol disalicylate and MTA. Dent Mater 29:1287–1294
Amoroso-Silva PA, Guimarães BM, Marciano MA, et al. (2014) Microscopic analysis of the quality of obturation and physical properties of MTA Fillapex. Microsc Res Tech 77:1031–1036
Assmann E, Böttcher DE, Hoppe CB, Grecca FS, Kopper PM (2015) Evaluation of bone tissue response to a sealer containing mineral trioxide aggregate. J Endod 41:62–66
Silva EJ, Perez R, Valentim RM, et al. (2016) Dissolution, dislocation and dimensional changes of endodontic sealers after a solubility challenge: a micro-CT approach. Int Endod J. doi:10.1111/iej.12636
Borges RP, Sousa-Neto MD, Versiani MA, et al. (2012) Changes in the surface of four calcium silicate-containing endodontic materials and an epoxy resin-based sealer after a solubility test. Int Endod J 45:419–428
Kim RJ, Shin JH (2014) Cytotoxicity of a novel mineral trioxide aggregate-based root canal sealer. Dent Mater J 33:313–318
Zoufan K, Jiang J, Komabayashi T, Wang YH, Safavi KE, Zhu Q (2011) Cytotoxic evaluation of Gutta Flow and EndoSequence BC sealers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 112:657–661
Candeiro GT, Moura-Netto C, D’Almeida-Couto RS, et al. (2015) Cytotoxicity, genotoxicity and antibacterial effectiveness of a bioceramic endodontic sealer. Int Endod J. doi:10.1111/iej.12523
Lim ES, Park YB, Kwon YS, Shon WJ, Lee KW, Min KS (2015) Physical properties and biocompatibility of an injectable calcium-silicate-based root canal sealer: in vitro and in vivo study. BMC Oral Health 21:129
Okiji T, Yoshiba K (2009) Reparative dentinogenesis induced by mineral trioxide aggregate: a review from the biological and physicochemical points of view. Int J Dent 2009:464280
Widbiller M, Lindner SR, Buchalla W, et al. (2016) Three-dimensional culture of dental pulp stem cells in direct contact to tricalcium silicate cements. Clin Oral Invest 20:237–246
International Organization for Standardization ISO 10993 Biological Evaluation of Medical Devices – Part 5 (2009) Tests for in vitro cytotoxicity. International Organization for Standardization, Geneva, Switzerland
Wrzesinski K, Magnone MC, Hansen LV, et al. (2013) HepG2/C3A spheroids exhibit stable physiological functionality for at least 24 days after recovering from trypsinisation. Toxicol Res 2:163–172
Silva EJ, Carvalho NK, Zaia AA (2016a) Cytotoxicity profile of epoxy resin sealer provided by a new 3D cell culture experimental model. ENDO 10:9–13
Schuster U, Schmalz G, Thonermann B, Mendel N, Metzl C (2001) Cytotoxicity testing with three-dimensional cultures of transfected pulp-derived cells. J Endod 27:259–265
De-Deus G, Canabarro A, Alves G, Linhares A, Senne MI, Granjeiro JM (2009) Optimal cytocompatibility of a bioceramic nanoparticlate cement in primary human mesenchymal cells. J Endod 35:1387–1390
Camps J, About I (2003) Cytotoxicity testing of endodontic sealers: a new method. J Endod 29:583–586
Seymour GJ, Gemmell E (2001) Cytokines in periodontal disease: where to from here? Acta Odont Scand 59:167–173
Ishimi Y, Miyaura C, Jin CH, et al. (1990) IL-6 is produced by osteoblasts and induces bone resorption. J Immunol 145:3297–3303
Ullm S, Krüger A, Tondera C, Gebauer TP, Neffe AT, Lendlein A, Jung F, Pietzsch J (2014) Biocompatibility and inflammatory response in vitro and in vivo to gelatin-based biomaterials with tailorable elastic properties. Biomaterials 35:9755–9766
Acknowledgments
Dr. Emmanuel JNL Silva is eligible with a JCNE grant from FAPERJ, Rio de Janeiro, Brazil. This study was partially funded by a FAPERJ grant E-26/010.001243/2015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
da Silva, E.J.N.L., Zaia, A.A. & Peters, O.A. Cytocompatibility of calcium silicate-based sealers in a three-dimensional cell culture model. Clin Oral Invest 21, 1531–1536 (2017). https://doi.org/10.1007/s00784-016-1918-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-016-1918-9