Abstract
Objectives
Different studies suggest that inflammation as well as hypoxia leads to an increase of p53 protein levels. However, the implication of p53 during oral inflammatory processes is still unknown. The aim of this study was therefore to investigate the effect of hypoxia and inflammation on p53 regulation in human periodontium in vitro and in vivo.
Materials and methods
Under hypoxic and normoxic conditions, human primary periodontal ligament (PDL) fibroblasts (n = 9) were stimulated with lipopolysaccharides (LPS) from Porphyromonas gingivalis (P.g.), a periodontal pathogenic bacterium. After different time points, cell viability was tested; p53 gene expression, protein synthesis, and activation were measured using quantitative RT-PCR, immunoblotting, and immunofluorescence. Moreover, healthy and inflamed periodontal tissues were obtained from 12 donors to analyze p53 protein in oral inflammatory diseases by immunohistochemistry.
Results
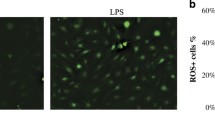
LPS-P.g. and hypoxia initially induced a significant upregulation of p53 mRNA expression and p53 protein levels. Nuclear translocation of p53 after inflammatory stimulation supported these findings. Hypoxia first enhanced p53 levels, but after 24 h of incubation, protein levels decreased, which was accompanied by an improvement of PDL cell viability. Immunohistochemistry revealed an elevation of p53 immunoreactivity in accordance to the progression of periodontal inflammation.
Conclusions
Our data indicate that p53 plays a pivotal role in PDL cell homeostasis and seems to be upregulated in oral inflammatory diseases.
Clinical relevance
Upregulation of p53 may promote the destruction of periodontal integrity. A possible relationship with carcinogenesis may be discussed.



Similar content being viewed by others
References
Brooks CL, Gu W (2006) p53 ubiquitination: Mdm2 and beyond. Mol. Cell 21:307–315
Vogelstein B, Lane D, Levine AJ (2000) Surfing the p53 network. Nature 408:307–310
Muller PA, Vousden KH (2013) p53 mutations in cancer. Nat. Cell Biol. 15:2–8
Stiewe T (2007) The p53 family in differentiation and tumorigenesis. Nat. Rev. Cancer 7:165–168
Maddocks OD, Vousden KH (2011) Metabolic regulation by p53. J Mol Med (Berl) 89:237–245
An WG, Kaneka M, Simon MC, Maltede E, Blagosklonny MV, Neckers LM (1998) Stabilization of wild-type p53 by hypoxia-inducible factor 1 alpha. Nature 392:405–208
Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM (2005) The antioxidant function of the p53 tumor suppressor. Nat. Med. 11:1306–1313
Bieging KT, Attardi LD (2012) Deconstructing p53 transcriptional networks in tumor suppression. Trends Cell Biol. 22:97–106
Vurusaner B, Poli G, Basaga H (2012) Tumor suppressor genes and ROS: complex networks of interactions. Free Radic. Biol. Med. 52:7–18
Gölz L, Memmert S, Rath-Deschner B, Jäger A, Appel T, Baumgarten G, Götz W, Frede S (2014) LPS from P. gingivalis and hypoxia increases oxidative stress in periodontal ligament fibroblasts and contributes to periodontitis. Mediat. Inflamm. 2014:986,264
Salvioli S, Capri M, Bucci L, Lanni C, Racchi M, Uberti D, Memo M, Mari D, Govoni S, Franceschi C (2009) Why do centenarians escape or postpone cancer? The role of IGF-1, inflammation and p53. Cancer Immunol. Immunother. 58:1909–1917
Salehinejad J, Zare-Mahmoodabadi R, Saghafi S, Jafarian AH, Ghazi N, Rajaei AR, Marouzi P (2011) Immunohistochemical detection of p53 and PCNA in ameloblastoma and adenomatoid odontogenic tumor. J. Oral Sci. 53:213–217
Galvão CF, Gomes CC, Diniz MG, Vargas PA, de Paula AM, Mosqueda-Taylor A, Loyola AM, Gomez RS (2012) Loss of heterozygosity (LOH) in tumour suppressor genes in benign and malignant mixed odontogenic tumours. J Oral Pathol Med 41:389–393
Aoyama I, Yaegaki K, Calenic B, Ii H, Ishkitiev N, Imai T (2012) The role of p53 in an apoptotic process caused by an oral malodorous compound in periodontal tissues: a review. J Breath Res 6:017104
Dai R, Iwama A, Wang S, Kapila YL (2005) Disease-associated fibronectin matrix fragments trigger anoikis of human primary ligament cells: p53 and c-myc are suppressed. Apoptosis 10:503–512
Ghosh A, Joo NE, Chen TC, Kapila YL (2010) Proapoptotic fibronectin fragment induces the degradation of ubiquitinated p53 via proteasomes in periodontal ligament cells. J. Periodontal Res. 45:481–487
Tonetti MS, Cortellini D, Lang NP (1998) In situ detection of apoptosis at sites of chronic bacterially induced inflammation in human gingiva. Infect. Immun. 66:5190–5195
Calenic B, Yaegaki K, Kozhuharova A, Imai T (2010) Oral malodorous compound causes oxidative stress and p53-mediated programmed cell death in keratinocyte stem cells. J. Periodontol. 81:1317–1323
Marchesan JT, Scanlon CS, Soehren S, Matsuo M, Kapila YL (2011) Implications of cultured periodontal ligament cells for the clinical and experimental setting: a review. Arch. Oral Biol. 56:933–943
Konermann A, Stabenow D, Knolle PA, Held SA, Deschner J, Jäger A (2012) Regulatory role of periodontal ligament fibroblasts for innate immune cell function and differentiation. Innate Immun 18:745–752
Thornton-Evans G, Eke P, Wei L, Palmer A, Moeti R, Hutchins S, Borrell LN (2013) Centers for Disease Control and Prevention (CDC). Periodontitis among adults aged ≥30 years - —United States, 2009–-2010. MMWR Surveill Summ 62 Suppl 3:129–-135
Costa FO, Cota LO, Lages EJ, Cyrino RM, Oliveira AM, Oliveira PA, Cortelli JR (2013) Associations of duration of smoking cessation and cumulative smoking exposure with periodontitis. J. Oral Sci. 55:245–253
Michalowicz BS, Aeppli D, Virag JG, Klump DG, Hinrichs JE, Segal NL, Bouchard TJ, Pihlstrom BL (1991) Periodontal findings in adult twins. J. Periodontol. 62:293–299
Mucci LA, Björkman L, Douglass CW, Pedersen NL (2005) Environmental and heritable factors in the etiology of oral diseases-—a population-based study of Swedish twins. J. Dent. Res. 84:800–805
Meyer MS, Joshipura K, Giovannucci E, Michaud DS (2008) A review of the relationship between tooth loss, periodontal disease, and cancer. Cancer Causes Control 19:895–907
Hosomi N, Aoki S, Matsuo K, Deguchi K, Masugata H, Murao K, Ichihara N, Ohyama H, Dobashi H, Nezu T, Ohtsuki T, Yasuda O, Soejima H, Ogawa H, Izumi Y, Kohno M, Tanaka J, Matsumoto M (2012) Association of serum anti-periodontal pathogen antibody with ischemic stroke. Cerebrovasc. Dis. 34:385–392
Hayashi C, Gudino CV, Gibson FC, Genco CA (2010) Review: pPathogen-induced inflammation at sites distant from oral infection: bacterial persistence and induction of cell-specific innate immune inflammatory pathways. Mol Oral Microbiol 25:305–316
Park HJ, Baek KH, Lee HL, Kwon A, Hwang HR, Qadir AS, Woo KM, Ryoo HM, Baek JH (2011) Hypoxia inducible factor-1α directly induces the expression of receptor activator of nuclear factor-κB ligand in periodontal ligament fibroblasts. Mol. Cell 31:573–578
Gölz L, Memmert S, Rath-Deschner B, Jäger A, Appel T, Baumgarten G, Götz W, Frede S (2015) Hypoxia and P. gingivalis sSynergistically iInduce HIF-1 and NF-κB aActivation in PDL cCells and pPeriodontal dDiseases. Mediat. Inflamm. 2015:438,085
Rath-Deschner B, Deschner J, Reimann S, Jager A, Gotz W (2009) Regulatory effects of biomechanical strain on the insulin-like growth factor system in human periodontal cells. J. Biomech. 42:2584–2589
Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent Jr RL (1998) Microbial complexes in subgingival plaque. J. Clin. Periodontol. 25:134–144
Hajishengallis G, Lamont RJ (2014) Breaking bad: manipulation of the host response by Porphyromonas gingivalis. Eur. J. Immunol. 44:328–338
Comel A, Sorrentino G, Capaci V, Del Sal G (2014) The cytoplasmic side of p53’s oncosuppressive activities. FEBS Lett. 58:2600–2609
Gamonal J, Bascones A, Acevedo A, Blanco E, Silva A (2001) Apoptosis in chronic adult periodontitis analyzed by in situ DNA breaks, electron microscopy, and immunohistochemistry. J. Periodontol. 72:517–525
Jarnbring F, Somogyi E, Dalton J, Gustafsson A, Klinge B (2002) Quantitative assessment of apoptotic and proliferative gingival keratinocytes in oral and sulcular epithelium in patients with gingivitis and periodontitis. J. Clin. Periodontol. 29:1065–1071
Bullon P, Fioroni M, Goteri G, Rubini C (2004) Battino M (2004) Immunohistochemical analysis of soft tissues in implants with healthy and peri-implantitis condition, and aggressive periodontitis. Clin Oral Implants Res 15:553–559
Bulut S, Uslu H, Ozdemir BH, Bulut OE (2006) Expression of caspase-3, p53 and Bcl-2 in generalized aggressive periodontitis. Head Face Med 2:17
Bascones A, Gamanol J, Gomez M, Silva A, Gonzalez MA (2004) New knowledge of the pathogenesis of periodontal disease. Quintessence Int. 35:706–716
Kebschull M, Guarnieri P, Demmer RT, Bouelsteix AL, Pavlidis P, Papapanou PN (2013) Molecular differences between chronic and aggressive periodontitis. J. Dent. Res. 92:1081
Lane DP (1992) Cancer. p53, guardian of the genome. Nature 358:15–16
Finkel T (2003) Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 15:247–254
Jackson AL, Loeb LA (2001) The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat. Res. 477:7–21
Macip S, Igarashi M, Berggren P, Yu J, Lee SW, Aaronson SA (2003) Influence of induced reactive oxygen species in p53-mediated cell fate decisions. Mol. Cell. Biol. 23:8576–8585
Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B (1997) A model for p53-induced apoptosis. Nature 389:300–305
Tan M, Li S, Swaroop M, Guan K, Oberley LW, Sun Y (1999) Transcriptional activation of the human glutathione peroxidase promoter by p53. J Biol Chem 274:12061–12066
Hussain SP, Amstad P, He P, Robles A, Lupold S, Kaneko I, Ichimiya M, Sengupta S, Mechanic L, Okamura S, Hofseth LJ, Moake M, Nagashima M, Forrester KS, Harris CC (2004) p53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Res. 64:2350–2356
Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM (2004) Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science 304:596–600
Yoon KA, Nakamura Y, Arakawa H (2004) Identification of ALDH4 as a p53-inducible gene and its protective role in cellular stresses. J. Hum. Genet. 49:134–140
Graeber TG, Peterson JF, Tsai M, Monica K, Fornace AJ, Giaccia AJ (1994) Hypoxia induces accumulation of p53 protein, but activation of a G1-phase checkpoint by low-oxygen conditions is independent of p53 status. Mol. Cell. Biol. 14:6264–6277
Acknowledgments
The authors like to thank Tina Schaffrath, Inka Müller-Bay, and Elisa Vestewig for their technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The present study was supported by a grant from the German Research Foundation (Clinical Research Unit 208/TP7, TP9) and the Medical Faculty of the University of Bonn.
Conflict of interests
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. A vote of approval by the ethics committee of the Faculty of Medicine at the University of Bonn was obtained (Lfd.Nr 086/11).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
S. Memmert, L. Gölz, S. Frede, and W. Götz contributed equally to this work.
Rights and permissions
About this article
Cite this article
Memmert, S., Gölz, L., Pütz, P. et al. Regulation of p53 under hypoxic and inflammatory conditions in periodontium. Clin Oral Invest 20, 1781–1789 (2016). https://doi.org/10.1007/s00784-015-1679-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-015-1679-x