Abstract
Objectives
To evaluate the enamel resistance and permeability of rat teeth to acid challenges after systemic use of sodium alendronate.
Materials and methods
Eighteen Wistar rats (36 teeth), aged 36–42 days (200–230 g), were assigned into two groups: alendronate group (n = 20 teeth), which received two weekly doses of 1 mg/kg of alendronate, via gavage; and a non-alendronate group (n = 16 teeth), which received only distilled water. After 60 days, the animals were killed, the maxillary incisors were extracted and used for the artificial induction of the caries lesion (pH cycling regimen) and erosion area (immersion cycles in cola-type soft drink) and for the enamel permeability test (dye penetration). The teeth were divided into alendronate group (n = 10) or non-alendronate group (n = 8) for each test. The quantitative response variables were the percent longitudinal change in Knoop microhardness (%LMC), the enamel carious/erosion lesion area (CELA) and enamel permeability.
Results
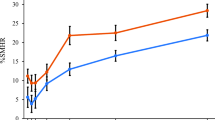
Groups were not significantly different (p > 0.05) with regard to the %LMC and enamel permeability (25.58 μm ± 12.73 and 25.40 μm ± 4.6 for the experimental and control groups, respectively). For CELA, it was not observed significant difference (p > 0.05) between the non-alendronate group (24.08 ± 1.36 and 25.22 ± 1.60, for caries and erosion, respectively) and the alendronate group (25.46 ± 1.60 and 25.0 ± 1.26) for caries and erosion, respectively).
Conclusions
Based on the methodological conditions and the presented results of this study, sodium alendronate did not affect the longitudinal microhardness, demineralisation lesion area or permeability of the enamel after acid challenge; therefore, alendronate did not become the enamel of rats more resistant.
Clinical relevance
The systemic alendronate treatment for 60 days did not change the enamel of rats regarding the susceptibility to acids.


Similar content being viewed by others
References
Gwinnett AJ (1992) Structure and composition of enamel. Oper Dent 5:10–17
Ten Cate AR (1994) Oral histology: development, structure and function, 4th edn. Mosby, St. Louis, MO
Gerlach RF, Cury JA, Krug FJ, Line RP (2002) Effect of lead on dental enamel formation. Toxicology 175:27–34
He LH, Swain MV (2009) Enamel—a functionally graded natural coating. J Dent 37:596–603
Turssi CP, Messias DF, Corona SM, Serra MC (2010) Viability of using enamel and dentin from bovine origin as a substitute for human counterparts in an intraoral erosion model. Braz Dent J 21:332–336
Wang LJ, Tang R, Bonstein T, Bush P, Nancollas GH (2006) Enamel demineralization in primary and permanent teeth. J Dent Res 85:359–363
Swan RM, Leite GA, Saraiva MC, Barbosa F Jr, Tanus-Santos JE, Gerlach RF (2010) Fluoride increases lead concentrations in whole blood and in calcified tissues from lead-exposed rats. Toxicology 271:21–26
Leite GA, Sawan RM, Teófilo JM, Porto IM, Sousa FB, Gerlach RF (2011) Exposure to lead exacerbates dental fluorosis. Arch Oral Biol 56:695–702
Massa LF, Bradaschia-Correa V, Arana-Chavez VE (2006) Immunocytochemical study of amelogenin deposition during the early odontogenesis of molars in alendronate-treated newborn rats. J Histochem Cytochem 54:713–725
Bradaschia-Correa V, Massa LF, Arana-Chavez VE (2007) Effects of alendronate on tooth eruption and molar root formation in young growing rats. Cell Tissue Res 330:475–485
do Espírito Santo AR, Frozoni MR, Ramos-Peres FM, Novaes PD, Line SR (2010) Birefringence of the secretory-stage enamel organic extracellular matrix from rats submitted to successive injections of bisphosphonates. Connect Tissue Res 51:208–215
Fleisch H (2007) Bisphosphonates—history and experimental basis. Bone 8(Suppl 1):S23–S28
Russell RGG, Watts NB, Ebetino FH, Rogers MJ (2008) Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int 19:733–759
Freundlich M, Alon US (2008) Bisphosphonates in children with hypercalciuria and reduced bone mineral density. Pediatr Nephrol 23:2215–2220
Mallet E (2008) Primary hyperparathyroidism in neonates and childhood. Horm Res 69:180–188
Bachrach LK, Ward LM (2009) Clinical review: bisphosphonate use in childhood osteoporosis. J Clin Endocrinol Metab 94:400–409
Ward LM, Rauch F, Whyte MP, D’Astous J, Gates PE, Grogan D, Lester EL, McCall RE, Pressly TA, Sanders JO, Smith PA, Steiner RD, Sullivan E, Tyerman G, Smith-Wright DL, Verbruggen N, Heyden N, Lombardi A, Glorieux FH (2011) Alendronate for the treatment of pediatric osteogenesis imperfecta: a randomized placebo-controlled study. J Clin Endocrinol Metab 96:355–356
Nelson-Filho P, Lucisano MP, Silva RAB, Silva RS, Serra MC, Gerlach RF, Rehder-Neto FC, Carneiro ZA, Zamarioli A, Morse L, Battaglino R (2012) Systemically alendronate was incorporated into dental tissues but did not cause morphological or mechanical changes in rats teeth. Microsc Res Tech 75:1265–1271
Keidel WD (1971) Physiology. Salvat, Barcelona
Amaechi BT, Higham SM (2001) In vitro remineralization of eroded enamel lesions by saliva. J Dent 29:371–376
Amaechi BT, Higham SM, Edgar WM (1999) Factors influencing the development of dental erosion in vitro: enamel type, temperature and exposure time. J Oral Rehabil 26:624–630
Pecora JD, Souza Neto MD, Costa WF (1991) Presentation of a chemical method which reveals the diffusion of 30% hydrogen peroxide through root dentin in vitro. Rev Paul Odontol 13:34–37 (In Portuguese)
Schiavoni RJ, Turssi CP, Rodrigues AL Jr, Serra MC, Pécora JD, Fröner IC (2006) Assessing the effect of bleaching agents on enamel permeability. Am J Dent 19:313–316
Attin T (2006) Methods for assessment of dental erosion. Monogr Oral Sci 20:152–172
Torres CP, Chinelatti MA, Gomes-Silva JM, Rizóli FA, Oliveira MA, Palma-Dibb RG, Borsatto MC (2010) Surface and subsurface erosion of primary enamel by acid beverages over time. Braz Dent J 21:337–345
Azevedo DT, Faraoni-Romano JJ, Derceli Jdos R, Palma-Dibb RG (2012) Effect of Nd:YAG laser combined with fluoride on the prevention of primary tooth enamel demineralization. Braz Dent J 23:104–109
Featherstone JD, ten Cate JM, Shariati M, Arends J (1983) Comparison of artificial caries-like lesions by quantitative microradiography and microhardness profiles. Caries Res 17:385–391
Jablonski-Momeni A, Ricketts DNJ, Stachniss V, Maschka R, Heinzel-Gutenbrunner M, Pieper K (2009) Occlusal caries: evaluation of direct microscopy versus digital imaging used for two histological classification systems. J Dent 37:204–211
Kaste SC, Hopkins KP, Jones D, Crom D, Greenwald CA, Santana VM (1997) Dental abnormalities in children treated for acute lymphoblastic leukemia. Leukemia 11:792–796
Gomes JR, Omar NF, Do Carmo ER, Neves JS, Soares MA, Narvaes EA, Novaes PD (2013) Relationship between cell proliferation and eruption rate in the rat incisor. Anat Rec (Hoboken) 296:1096–1101
Gandolfi MG, Nucci C, Prati C, Mongiorgi R (2007) Dental enamel dissolution after alendronate treatment. Am J Dent 20:235–240
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Compliance with ethical standards
The study was approved by the institutional Ethics Committee for Animal Care and Research Use (Protocol 10.1.468.53.5).
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Nelson-Filho, P., Rossi, C.R.B., Gomes-Silva, J.M. et al. Enamel permeability and resistance to acid challenges after systemic use of sodium alendronate: a study in rat teeth. Clin Oral Invest 20, 1647–1654 (2016). https://doi.org/10.1007/s00784-015-1647-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-015-1647-5