Abstract
Objectives
The purpose of this in vitro study was to determine the antimicrobial activity of two different taurolidine gel formulations in comparison with minocycline microspheres.
Methods
Three percent taurolidine gel (TLG3) and 2 % taurolidine gel (TLG2) were compared to minocycline microspheres (MINO) against single bacterial species and a 12-species-mixture. The antimicrobial activity was proven by determination of minimal inhibitory concentrations (MICs), killing assays, after exposure of the antimicrobials as well as within a biofilm.
Results
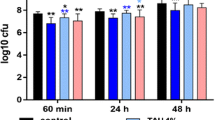
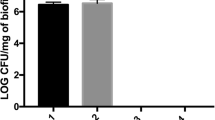
The MICs against the single species were between 0.5 and 2 mg/ml of taurolidine. MICs of the used mixed microbiota were 1.5 mg/ml (TLG3) and 4 mg/ml (TLG2). Fusobacterium nucleatum and Porphyromonas gingivalis were completely killed by 10 % TLG3 and TLG2 (equivalent to 3 and 2 mg/ml taurolidine) after 6 h. The mixture of 12 species was not completely killed by any of the test substances. Taurolidine gels showed a post-antimicrobial activity, however being less than that of MINO. On biofilms, taurolidine gels reduced concentration dependently the colony forming unit (CFU) counts (multi-species biofilms by 3.63 log10 after 100 % (30 mg/ml) of TLG3), reductions were 2.12 log10 after MINO (1000 μg/ml minocycline).
Conclusions
Taurolidine gel formulations exert antimicrobial activity against bacteria associated with periodontal disease. Nevertheless, a complete elimination of biofilms seems to be impossible and underlines the importance of mechanical removal of biofilms prior to application of the antimicrobial.
Clinical relevance
Taurolidine gels may represent a potential alternative for adjunctive topical antimicrobial treatment in periodontitis and infectious peri-implant diseases.




Similar content being viewed by others
References
Gidley MJ, Sanders JK, Myers ER, Allwood MC (1981) The mode of antibacterial action of some ‘masked’ formaldehyde compounds. FEBS Lett 127:225–227
Caruso F, Darnowski JW, Opazo C, Goldberg A, Kishore N, Agoston ES, Rossi M (2010) Taurolidine antiadhesive properties on interaction with E. coli; its transformation in biological environment and interaction with bacteria cell wall. PLoS One 5:e8927
Schuller-Levis GB, Park E (2003) Taurine: new implications for an old amino acid. FEMS Microbiol Lett 226:195–202
Linder MM, Ott W, Wesch G, Wicki O, Marti MC, Moser G (1981) Therapy of purulent peritonitis. Documentation of 78 cases and experience with taurolin (author’s transl). Langenbecks Arch Chir 353:241–250
Arweiler NB, Auschill TM, Sculean A (2012) Antibacterial effect of taurolidine (2%) on established dental plaque biofilm. Clin Oral Investig 16:499–504
John G, Schwarz F, Becker J (2015) Taurolidine as an effective and biocompatible additive for plaque-removing techniques on implant surfaces. Clin Oral Investig 19:1069–1077
John G, Becker J, Schwarz F (2014) Effects of taurolidine and chlorhexidine on SaOS-2 cells and human gingival fibroblasts grown on implant surfaces. Int J Oral Maxillofac Implants 29:728–734
Eick S, Radakovic S, Pfister W, Nietzsche S, Sculean A (2012) Efficacy of taurolidine against periodontopathic species—an in vitro study. Clin Oral Investig 16:735–744
Reynolds S, Moran J, Addy M, Wade WG, Newcombe R (1991) Taurolin as an oral rinse. I. Antimicrobial effects in vitro and in vivo. Clin Prev Dent 13:13–17
Blenkharn JI (1990) In-vitro antibacterial activity of noxythiolin and taurolidine. J Pharm Pharmacol 42:589–590
Tew JG, Marshall DR, Burmeister JA, Ranney RR (1985) Relationship between gingival crevicular fluid and serum antibody titers in young adults with generalized and localized periodontitis. Infect Immun 49:487–493
Zollinger L, Schnyder S, Nietzsche S, Sculean A, Eick S (2015) In-vitro activity of taurolidine on single species and a multispecies population associated with periodontitis. Anaerobe 32:18–23
Goodson JM (2003) Gingival crevice fluid flow. Periodontol 2000(31):43–54
Pradeep AR, Sagar SV, Daisy H (2008) Clinical and microbiologic effects of subgingivally delivered 0.5% azithromycin in the treatment of chronic periodontitis. J Periodontol 79:2125–2135
Stoller NH, Johnson LR, Trapnell S, Harrold CQ, Garrett S (1998) The pharmacokinetic profile of a biodegradable controlled-release delivery system containing doxycycline compared to systemically delivered doxycycline in gingival crevicular fluid, saliva, and serum. J Periodontol 69:1085–1091
Van Dyke TE, Offenbacher S, Braswell L, Lessem J (2002) Enhancing the value of scaling and root-planing: arestin clinical trial results. J Int Acad Periodontol 4:72–76
Kim TS, Burklin T, Schacher B, Ratka-Kruger P, Schaecken MT, Renggli HH, Fiehn W, Eickholz P (2002) Pharmacokinetic profile of a locally administered doxycycline gel in crevicular fluid, blood, and saliva. J Periodontol 73:1285–1291
Olthof ED, Rentenaar RJ, Rijs AJ, Wanten GJ (2013) Absence of microbial adaptation to taurolidine in patients on home parenteral nutrition who develop catheter related bloodstream infections and use taurolidine locks. Clin Nutr 32:538–542
Saunders J, Naghibi M, Leach Z, Parsons C, King A, Smith T, Stroud M (2015) Taurolidine locks significantly reduce the incidence of catheter-related blood stream infections in high-risk patients on home parenteral nutrition. Eur J Clin Nutr 69:282–284
Handrup MM, Fuursted K, Funch P, Moller JK, Schroder H (2012) Biofilm formation in long-term central venous catheters in children with cancer: a randomized controlled open-labelled trial of taurolidine versus heparin. APMIS 120:794–801
Zwiech R, Adelt M, Chrul S (2013) A taurolidine-citrate-heparin lock solution effectively eradicates pathogens from the catheter biofilm in hemodialysis patients. Am J Ther. doi:10.1097/MJT.0b013e31828d4610
Lessem J, Hanlon A (2004) A post-marketing study of 2805 patients treated for periodontal disease with Arestin. J Int Acad Periodontol 6:150–153
Paquette DW, Hanlon A, Lessem J, Williams RC (2004) Clinical relevance of adjunctive minocycline microspheres in patients with chronic periodontitis: secondary analysis of a phase 3 trial. J Periodontol 75:531–536
Matesanz-Perez P, Garcia-Gargallo M, Figuero E, Bascones-Martinez A, Sanz M, Herrera D (2013) A systematic review on the effects of local antimicrobials as adjuncts to subgingival debridement, compared with subgingival debridement alone, in the treatment of chronic periodontitis. J Clin Periodontol 40:227–241
Sherertz RJ, Boger MS, Collins CA, Mason L, Raad II (2006) Comparative in vitro efficacies of various catheter lock solutions. Antimicrob Agents Chemother 50:1865–1868
Shchipkova AY, Nagaraja HN, Kumar PS (2010) Subgingival microbial profiles of smokers with periodontitis. J Dent Res 89:1247–1253
Kolenbrander PE, Palmer RJ Jr, Periasamy S, Jakubovics NS (2010) Oral multispecies biofilm development and the key role of cell-cell distance. Nat Rev Microbiol 8:471–480
Hoiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O (2010) Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 35:322–332
Noiri Y, Okami Y, Narimatsu M, Takahashi Y, Kawahara T, Ebisu S (2003) Effects of chlorhexidine, minocycline, and metronidazole on Porphyromonas gingivalis strain 381 in biofilms. J Periodontol 74:1647–1651
Shah CB, Mittelman MW, Costerton JW, Parenteau S, Pelak M, Arsenault R, Mermel LA (2002) Antimicrobial activity of a novel catheter lock solution. Antimicrob Agents Chemother 46:1674–1679
Tsaousoglou P, Nietzsche S, Cachovan G, Sculean A, Eick S (2014) Antibacterial activity of moxifloxacin on bacteria associated with periodontitis within a biofilm. J Med Microbiol 63:284–292
Reynolds S, Moran J, Wade WG, Addy M, Newcombe R (1991) Taurolin as an oral rinse. II. Effects on in vitro and in vivo plaque regrowth. Clin Prev Dent 13:18–22
Olthof ED, Nijland R, Gulich AF, Wanten GJ (2015) Microbiocidal effects of various taurolidine containing catheter lock solutions. Clin Nutr 34:309–314
Larsen T, Fiehn NE (1997) Development of resistance to metronidazole and minocycline in vitro. J Clin Periodontol 24:254–259
Nakao R, Takigawa S, Sugano N, Koshi R, Ito K, Watanabe H, Senpuku H (2011) Impact of minocycline ointment for periodontal treatment of oral bacteria. Jpn J Infect Dis 64:156–160
Braumann C, Guenther N, Pohlenz J, Pfirrmann RW, Menenakos C (2009) Wound healing is not impaired in rats undergoing perioperative treatment with the antineoplastic agent taurolidine. Eur Surg Res 42:91–96
Hartel C, Scholz T, Kuhn M, Bendiks M, Gopel W, Lauten M, Herting E (2013) Innate immune responses to Stenotrophomonas maltophilia in immunocompromised pediatric patients and the effect of taurolidine. J Microbiol Immunol Infect 46:115–120
Acknowledgments
The authors would like to thank Marianne Weibel (Department of Periodontology, Laboratory of Oral Microbiology, School of Dental Medicine, University of Bern) for technical assistance.
Conflict of interest
This study was funded by Geistlich Pharma AG, Wolhusen, Switzerland. Jürg Zumbrunn is an employee of Geistlich Pharma AG. The other authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eick, S., Gloor, N., Püls, C. et al. In vitro activity of taurolidine gel on bacteria associated with periodontitis. Clin Oral Invest 20, 597–606 (2016). https://doi.org/10.1007/s00784-015-1549-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-015-1549-6