Abstract
Objectives
Recurrent aphthous stomatitis (RAS) is the most common oral mucosal disease. Despite plenty of studies on aetiopathogenesis of RAS, a definite cause is not clear. The objective of this study was to determine the potential changes of salivary IgA and salivary flow rate in patients affected with minor form of RAS.
Materials and methods
Levels of salivary IgA in 33 patients with acute RAS (minor form) and 33 matched healthy controls were determined using enzyme-linked immunosorbent assay. Resting salivary flow rates were determined too. Both measurements, levels of salivary IgA and resting salivary flow rate, were performed again for each RAS patient in remission phase.
Results
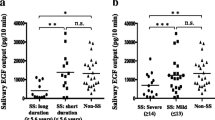
Levels of salivary IgA were significantly increased in acute phase of RAS [median (interquartile range)—124.94 μg/mL (106.22–136.31)] in comparison with the levels in healthy controls [88.92 μg/mL (76.85–93.91; P < 0.001)] and with the levels in remission phase [102.4 μg/mL (84.6–120.16; P = 0.01)]. Even in the disease-free period (remission phase), levels of salivary IgA remained significantly higher in comparison with the levels in healthy controls (P = 0.01). Salivary flow rates, on the other side, were not influenced by the disease state (RAS vs. healthy), phase (acute vs. remission) or even gender (males vs. females).
Conclusion
Marked increase of salivary IgA in acute and remission phases of the minor RAS may suggest a potential role for this immunoglobulin in pathogenesis of the disease.
Clinical relevance
Salivary IgA may be an important aetiological agent in the pathogenesis of RAS, and hence, its immunomodulation may help prevent the disease.
Similar content being viewed by others
References
Chattopadhyay A, Shetty KV (2011) Recurrent aphthous stomatitis. Otolaryngol Clin North Am 44(1):79–88. doi:10.1016/j.otc.2010.09.003
Natah SS, Konttinen YT, Enattah NS, Ashammakhi N, Sharkey KA, Hayrinen-Immonen R (2004) Recurrent aphthous ulcers today: a review of the growing knowledge. Int J Oral Maxillofac Surg 33(3):221–234. doi:10.1006/ijom.2002.0446
Jurge S, Kuffer R, Scully C, Porter SR (2006) Mucosal disease series. Number VI. Recurrent aphthous stomatitis. Oral Dis 12(1):1–21. doi:10.1111/j.1601-0825.2005.01143.x
Scully C, Porter S (2008) Oral mucosal disease: recurrent aphthous stomatitis. Br J Oral Maxillofac Surg 46(3):198–206. doi:10.1016/j.bjoms.2007.07.201
Preeti L, Magesh K, Rajkumar K, Karthik R (2011) Recurrent aphthous stomatitis. J Oral Maxillofac Pathol 15(3):252–256. doi:10.4103/0973-029X.86669
Baccaglini L, Lalla RV, Bruce AJ, Sartori-Valinotti JC, Latortue MC, Carrozzo M, Rogers RS III (2011) Urban legends: recurrent aphthous stomatitis. Oral Dis 17(8):755–770. doi:10.1111/j.1601-0825.2011.01840.x
Kumar BP, Keluskar V, Bagewadi AS, Shetti A (2010) Evaluating and comparing phagocytic functions of salivary and blood neutrophils in patients with recurrent aphthous ulcers and controls. Quintessence Int 41(5):411–416
Natah SS, Hayrinen-Immonen R, Hietanen J, Malmstrom M, Konttinen YT (2000) Immunolocalization of tumor necrosis factor-alpha expressing cells in recurrent aphthous ulcer lesions (RAU). J Oral Pathol Med 29(1):19–25
Natah SS, Hayrinen-Immonen R, Hietanen J, Patinen P, Malmstrom M, Savilahti E, Konttinen YT (2000) Increased density of lymphocytes bearing gamma/delta T-cell receptors in recurrent aphthous ulceration (RAU). Int J Oral Maxillofac Surg 29(5):375–380
Sistig S, Vucicevic-Boras V, Lukac J, Kusic Z (2002) Salivary IgA and IgG subclasses in oral mucosal diseases. Oral Dis 8(6):282–286
Martinez Kde O, Mendes LL, Alves JB (2007) Secretory A immunoglobulin, total proteins and salivary flow in Recurrent Aphthous Ulceration. Braz J Otorhinolaryngol 73(3):323–328
Russmann H, Lissner R, Schmidt H, Karch H (1999) IgA/IgM and secretory immunity. Sepsis 3:219–224
Brandtzaeg P (2007) Do salivary antibodies reliably reflect both mucosal and systemic immunity? Ann NY Acad Sci 1098(3):288–311
Marcotte H, Lavoie MC (1998) Oral microbial ecology and the role of salivary immunoglobulin A. Microbiol Mol Biol Rev 62(1):71–109
Ben-Aryeh H, Malberger E, Gutman D, Szargel R, Anavi Y (1976) Salivary IgA and serum IgG and IgA in recurrent aphthous stomatitis. Oral Surg Oral Med Oral Pathol 42(6):746–752
Brozovic S, Vucicevic-Boras V, Bukovic D (2001) Serum IgA, IgG, IgM and salivary IgA in recurrent aphthous ulceration. Coll Antropol 25(2):633–637
Saluja R, Kale A, Hallikerimath S (2012) Determination of levels of salivary IgA subclasses in patients with minor recurrent aphthous ulcer. J Oral Maxillofac Pathol 16:49–53
Lehner T (1969) Immunoglobulin estimation of blood and saliva in human recurrent oral ulceration. Arch Oral Biol 14(4):351–364
Bennet KR, Reade PC (1982) Salivary immunoglobulin A levels in normal subjects, tobacco smokers and patients with minor aphthous ulceration. Oral Surg Oral Med Oral Pathol 53:461–465
Abbas AK, Lichtman AH (2003) Cellular and molecular immunology, 5th edn. Saunders, China
Schett G, Metzler B, Kleindienst R (1997) Salivary anti-hsp65 antibodies as a diagnostic marker for gingivitis and a possible link to atherosclerosis. Int Arch Allergy Immunol 114:246–250
Donatsky O (1975) An immunofluorescence study on the cross-reaction between Strep. 2a and human oral mucosa. Scand J Dent Res 83(2):111–119
Taubman MA, Smith DJ (1993) Significance of salivary antibody in dental disease. Ann N Y Acad Sci 694:202–215
Rashkova MP, Toncheva AA (2010) Gingival disease and secretory immunoglobulin A in non-stimulated saliva in children. Folia Med 52(4):48–55
Henskens YM, van den Keijbus PA, Veerman EC (1996) Protein composition of whole and parotid saliva in healthy and periodontitis subjects: determination of cystatins, albumin, amylase and IgA. J Periodont Res 31(7):57–65
Hagewald S, Bernimoulin J-P, Kottgen E, Kage A (2002) Salivary IgA subclasses and bacteria-reactive IgA in patients with aggressive periodontitis. J Periodont Res 37:333–339
Ozdemir I, Calka O, Karadag A, Akdeniz N, Ozturk M (2011) Thyroid autoimmunity associated with recurrent aphthous stomatitis. J Eur Acad Dermatol Venereol. doi:10.1111/j.1468-3083.2011.04040.x
Farnaud SJC, Kosti O, Getting SJ, Renshaw D (2010) Saliva: physiology and diagnostic potential in health and disease. Sci World J 10:434–456
Wu-Wang CY, Patel M, Feng J, Milles M, Wang SL (1995) Decreased levels of salivary prostaglandin E2 and epidermal growth factor in recurrent aphthous stomatitis. Arch Oral Biol 40(12):1093–1098
Acknowledgments
The results presented in this study are part of the first author’s master project which was financially supported by the Faculty of Dentistry, Damascus University.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohammad, R., Halboub, E., Mashlah, A. et al. Levels of salivary IgA in patients with minor recurrent aphthous stomatitis: a matched case–control study. Clin Oral Invest 17, 975–980 (2013). https://doi.org/10.1007/s00784-012-0785-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-012-0785-2