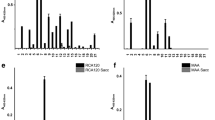
Abstract
Since the adhesion of bacteria to the tooth surface is a prerequisite for dental plaque and subsequent caries development, a promising caries preventive strategy could be to block the lectin–glycan-mediated adherence of cariogenic bacteria. The aim of the study was to evaluate potential differences in glycan-binding specificities of two Streptococcus mutans strains (DSM 20523 and DSM 6178) and Streptococcus sobrinus (DSM 20381). A competitive enzyme-linked lectin-binding assay was used to identify the binding specificities of isolated bacterial surface lectins. Blotting of the microbial proteins on neoglycoprotein-coated PVP membranes enabled a qualitative protein analysis of all specific bacterial lectins. Different glycan-binding sites could be identified for the S. mutans strains in comparison to S. sobrinus. An earlier reported glycan-binding specificity for terminal galactose residues could be confirmed for the S. mutans strains. For the S. sobrinus strain, more than one glycan-binding specificity could be found (oligomannose and terminal sialyl residues). Each of the tested strains showed more than one surface lectin responsible for the specific lectin-binding with varying molecular weight (S. mutans, 90/155 kDa and S. sobrinus, 35/45 kDa). The established experimental setup could be used as future standard procedure for the identification of bacterial lectin-derived binding specificities. The findings from this study might serve as basis for the design of an individual ‘glycan cocktail’ for the competitive inhibition of lectin-mediated adhesion of mutans streptococci to oral surfaces.




Similar content being viewed by others
References
Nurelhuda NM, Al-Haroni M, Trovik TA, Bakken V (2010) Caries experience and quantification of Streptococcus mutans and Streptococcus sobrinus in saliva of Sudanese schoolchildren. Caries Res 44:402–407
Choi EJ, Lee SH, Kim YJ (2009) Quantitative real-time polymerase chain reaction for Streptococcus mutans and Streptococcus sobrinus in dental plaque samples and its association with early childhood caries. Int J Paediatr Dent 19:141–147
Loesche WJ (1986) Role of Streptococcus mutans in human dental decay. Microbiol Rev 50:353–380
Fitzgerald RJ, Keyes PH (1960) Demonstration of the etiologic role of streptococci in experimental caries in the hamster. J Am Dental Assoc 61:9–19
Matsumura M, Izumi T, Matsumoto M, Tsuji M, Fujiwara T, Ooshima T (2003) The role of glucan-binding proteins in the cariogenicity of Streptococcus mutans. Microbiol Immunol 47:213–215
Koo H, Xiao J, Klein MI (2009) Extracellular polysaccharide matrix—an often forgotten virulence factor in oral biofilm research. Int J Oral Sci 1:229–234
Ericsson Y (1978) Progress in caries prevention. Caries Res 12(Suppl):1–112
Micheelis W, Schiffner U (2006) Vierte Deutsche Mundgesundheitsstudie (DMS IV). Neue Ergebnisse zu oralen Erkrankungsprävalenzen, Risikogruppen und zum zahnärztlichen Versorgungsgrad in Deutschland 2005, vol. 31. Deutscher Zahnärzte Verlag
Marthaler TM (2004) Changes in dental caries 1953–2003. Caries Res 38:173–181
Gibbons RJ (1989) Bacterial adhesion to oral tissues: a model for infectious diseases. J Dental Res 68:750–760
Kelly CG, Younson JS (2000) Anti-adhesive strategies in the prevention of infectious disease at mucosal surfaces. Expert Opin Invest Drugs 9:1711–1721
Ofek I, Hasty DL, Sharon N (2003) Anti-adhesion therapy of bacterial diseases: prospects and problems. FEMS Immunol Med Microbiol 38:181–191
Sharon N (2006) Carbohydrates as future anti-adhesion drugs for infectious diseases. Biochim Biophys Acta 1760:527–537
Kage A, Fimmel S, Bernimoulin JP, Hagewald S, Nitschke I, Kage R, Kottgen E (1995) Oligosaccharides in mucosal host defense: model, method, and first data. Adv Exp Med Biol 371B:1177–1182
Seemann R, Zimmer S, Bizhang M, Kage A (2001) Differences in the salivary glycan pattern between children with high and low caries susceptibility. Caries Res 35:156–161
Kage A, Weitzel D, Köttgen E (1989) Novel competitive lectin-binding inhibition assay for quantitative characterization of glycoconjugates using different peroxidase labeled lectins. J Clin Chem Clin Biochem 27:701–702
Wu AM, Song SC, Tsai MS, Herp A (2001) A guide to the carbohydrate specificities of applied lectins-2 (updated in 2000). Adv Exper Med Biol 491:551–585
Levine MJ, Tabak LA, Reddy MS, Mandel ID (1985) Nature of salivary pellicles in microbial adherence: role of salivary mucins. In: Mergenhagen SE, Rosan B (eds) Molecular basis of oral microbiol adhesion. American Society for Microbiology, Washington, pp 125–130
Zehetbauer S, Wojahn T, Hiller KA, Schmalz G, Ruhl S (2009) Resemblance of salivary protein profiles between children with early childhood caries and caries-free controls. Eur J Oral Sci 117:369–373
Drews J (2005) Konzentration Lektin-spezifischer Speichelglykane im Verlauf einer experimentellen Gingivits. Zahnmedizinische Dissertation, Zentrum für Zahnmedizin Campus Virchow-Klinikum der Medizinischen Fakultät der Charité, Berlin
Aronson M, Medalia O, Schori L, Mirelman D, Sharon N, Ofek I (1979) Prevention of colonization of the urinary tract of mice with Escherichia coli by blocking of bacterial adherence with methyl alpha-d-mannopyranoside. J Infect Dis 139:329–332
Steinberg D, Feldman M, Ofek I, Weiss EI (2004) Effect of a high-molecular-weight component of cranberry on constituents of dental biofilm. J Antimicrob Chemother 54:86–89
Weiss EI, Kozlovsky A, Steinberg D, Lev-Dor R, Bar Ness Greenstein R, Feldman M, Sharon N, Ofek I (2004) A high molecular mass cranberry constituent reduces mutans streptococci level in saliva and inhibits in vitro adhesion to hydroxyapatite. FEMS Microbiol Lett 232:89–92
Seemann R, Kluck I, Kage A (2006) An in vitro microbial-based model for studying caries-preventive agents. Acta Odontol Scand 64:27–30
Gibbons RJ, Qureshi JV (1979) Inhibition of adsorption of Streptococcus mutans strains to saliva-treated hydroxyapatite by galactose and certain amines. Infect Immun 26:1214–1217
Nagata K, Shibata S, Inoshita E, Tamagawa H, Shizukuishi E, Tsunemitsu A (1982) The effect of daily mouth rinsing with galactose on dental plaque formation. J Dent Health 32:104–107
Levine MJ, Herzberg MC, Levine MS, Ellison SA, Stinson MW, Li HC, van Dyke T (1978) Specificity of salivary-bacterial interactions: role of terminal sialic acid residues in the interaction of salivary glycoproteins with Streptococcus sanguis and Streptococcus mutans. Infect Immun 19:107–115
Ligtenberg AJ, Veerman EC, de Graaff J, Nieuw Amerongen AV (1990) Saliva-induced aggregation of oral streptococci and the influence of blood group reactive substances. Arch Oral Biol 35:141S–143S
Gibbons RJ, Cohen L, Hay DI (1986) Strains of Streptococcus mutans and Streptococcus sobrinus attach to different pellicle receptors. Infect Immun 52:555–561
Murray PA, Prakobphol A, Lee T, Hoover CI, Fisher SJ (1992) Adherence of oral streptococci to salivary glycoproteins. Infect Immun 60:31–38
Conflicts of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schüler, V., Lussi, A., Kage, A. et al. Glycan-binding specificities of Streptococcus mutans and Streptococcus sobrinus lectin-like adhesins. Clin Oral Invest 16, 789–796 (2012). https://doi.org/10.1007/s00784-011-0568-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-011-0568-1