Abstract
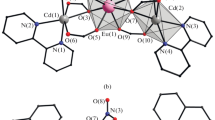
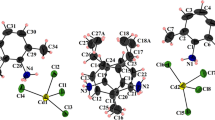
Infrared spectra of Cd(propylenediamine) Ni(CN)4 ·1,5benzene and M(propylenediamine)Ni(CN)4 (M = Co or Ni) are reported. Their spectral data were compared with those of the corresponding hosts. Clathrate and complex structures are different from each other. The complex (host) structures consist of corrugated polymeric layers of {M-Ni(CN)4}∞ with the propylenediamine molecules with the N-ends bound (chelated) to the metal M (M = Co or Ni). The spectral features suggest that the clathrate compounds are similar in structure to the Hofmann -pn-type clathrates.
Similar content being viewed by others
References
Akyüz S, Dempster AB, Morehouse L (1974) Host-guest interactions and stability of Hofmann-type benzene and aniline clathrates studied by i.r. spectroscopy. Spectrochim Acta 30A: 1989–2004
Bayrak C, Kantarcı Z (1997) Vibrational spectroscopic studies on the tn-Td-type Mn(tn)Zn(CN)4 · 2C6H6 and the chelated tn-Td-type Zn(tn)Zn(CN)4 · 2C6H6 clathrates. J Incl Phenom: in press
Davies JED, Dempster AB, Suzuki S (1974) Clathrate and inclusion compounds-II(!). The Raman spectra of Hofmann-type benzene and benzene-D6 clathrates, Spectrochim Acta 30: 1188–1192
Kasap E, Kantarcı Z (1995) Vibrational spectroscopic studies on the en-Td-type benzene clathrates: M(ethylenediamine) M′(CN)4 · 2C6H6 (M = Mn or Cd; M′ = Cd or Hg). J Incl Phenom 23: 1–9
Kasap E, Özçelik S (1997) Vibrational spectroscopic studies on the Hofmann-dabn-type benzene clathrates: M(1,4-diaminobutane) Ni(CN)4 ·1,5C6H6 (M = Mn, Fe, Co, Ni or Cd). J Incl Phenom 28: 259–267
McCullough RL, Jones LH, Crosby GA (1960) An analysis of the vibrational spectrum of the tetracyanonickelate(II) ion in a crystal lattice. Spectrochim, Acta 16: 929–944
Nishikiori S, Iwamoto T, Yoshino Y (1980) Three-dimensional metal complex structures with ambident propylenediamine ligands serving as the hosts of the aromatic guest molecules. Hofmann-pn and pn-Td type clathrates. Bull Chem Soc Jpn 53: 2236–2240
Painter PC, Koening JL (1977) A normal vibrational analysis of benzene. Spectrochim Acta 33A: 1019–1023
Park K, Iwamoto T (1992) Urea- and Thiourea-like host structures of catena-[(1,2-diaminopropane)-cadmium(II) tetra-μ-cyano-nickelate(II)] accommodating aliphatic guests. J Chem Soc Chem Commun: 72–74
Park K, Iwamoto T (1993) The metal complex host catena-[(1,2-diaminopropane)-cadmium(II) tetracyanonickelate(II)] which provides channel cavities for straight- and branched-chain aliphatic guests. J Chem Soc Dalton Trans: 1785–1881
Park K, Hashimoto M, Kitazawa T, Iwamoto T (1990) Novel three-dimensional metal-complex host structures of catena-[propylenediaminecadmium(II) tetra-μ-cyanonickelate(II)] and catena-[propylenediaminecadmium(II) tetra-μ-cyanocadmate (II)]. Chem Lett: 1701–1704
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bayrak, C., Kasap, E. & Kantarci, Z. Infrared spectroscopic study of the Hofmann-pn-type clathrates: Cd(propylenediamine)Ni(CN)4 · 1,5benzene and M(propylenediamine) Ni(CN)4 (M=Co or Ni). ARI 50, 214–216 (1997). https://doi.org/10.1007/s007770050017
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s007770050017