Abstract
Purpose
Degenerative scoliosis (DS) is an important degenerative lumbar disease causing spinal dysfunction. The true reason or pathogenesis of DS is still unknown. Bone marrow-derived mesenchymal stem cells (BM-MSCs) are the stem/progenitor cells of the osteoblasts. The diseases associated with osteogenesis could be caused by abnormality of the MSCs. The purpose of this study was to find the differential proteins expressed in MSCs of patients with DS.
Methods
We collected and cultured the MSCs from 12 DS patients and 12 age- and gender-matched patients with lumbar spinal stenosis. Then the MSC samples were analyzed with 2D-DIGE and MALDI-TOF–MS to find the differential proteins which were further validated by Western blot.
Results
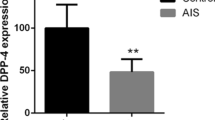
We found 115 spots that were differently expressed in the MSC of DS patients with 2D-DIGE, and 44 proteins were identified from samples of DS and control using MALDI-TOF–MS. Of these proteins, PIAS2, NDUFA2, and TRIM 68, which were up-regulated in DS more than 4 times were validated by Western blot.
Conclusions
The information obtained with this proteomics analysis will be useful in understanding the pathophysiology of DS. Further investigations on the functioning pathway, the specificity and the mechanism of these proteins will be carried out.




Similar content being viewed by others
References
Faldini C, Pagkrati S, Grandi G, Digennaro V, Faldini O, Giannini S. Degenerative lumbar scoliosis: features and surgical treatment. J Orthop Traumatol. 2006;7:67–71.
Pappou IP, Girardi FP, Sandhu HS, Parvataneni HK, Cammisa FP Jr, Schneider R, Frelinghuysen P, Lane JM. Discordantly high spinal bone mineral density values in patients with adult lumbar scoliosis. Spine (Phila Pa 1976). 2006; 31:1614–20.
Daffner SD, Vaccaro AR. Adult degenerative lumbar scoliosis. Am J Orthop (Belle Mead NJ). 2003; 32:77–82 (discussion 82).
Tribus CB. Degenerative lumbar scoliosis: evaluation and management. J Am Acad Orthop Surg. 2003;11:174–83.
Zhu Y, Han S, Zhao H, Liang J, Zhai J, Wu Z, Qiu G. Comparative analysis of serum proteomes of degenerative scoliosis. J Orthop Res. 2011;29:1896–903.
Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11:1198–211.
Bielby R, Jones E, McGonagle D. The role of mesenchymal stem cells in maintenance and repair of bone. Injury. 2007;38(Suppl 1):S26–32.
Shirley D, Marsh D, Jordan G, McQuaid S, Li G. Systemic recruitment of osteoblastic cells in fracture healing. J Orthop Res. 2005;23:1013–21.
Zhang W, Ge W, Li C, You S, Liao L, Han Q, Deng W, Zhao RC. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004;13:263–71.
Zhou H, Guo M, Bian C, Sun Z, Yang Z, Zeng Y, Ai H, Zhao RC. Efficacy of bone marrow-derived mesenchymal stem cells in the treatment of sclerodermatous chronic graft-versus-host disease: clinical report. Biol Blood Marrow Transplant. 2010;16:403–12.
Wagner W, Feldmann RE Jr, Seckinger A, Maurer MH, Wein F, Blake J, Krause U, Kalenka A, Bürgers HF, Saffrich R, Wuchter P, Kuschinsky W, Ho AD. The heterogeneity of human mesenchymal stem cell preparations—evidence from simultaneous analysis of proteomes and transcriptomes. Exp Hematol. 2006;34:536–48.
Ramagli LS. Quantifying protein in 2-D PAGE solubilization buffers. Methods Mol Biol. 1999;112:99–103.
Shuai K, Liu B. Regulation of gene-activation pathways by PIAS proteins in the immune system. Nat Rev Immunol. 2005;5:593–605.
Rosas-Acosta G, Langereis MA, Deyrieux A, Wilson VG. Proteins of the PIAS family enhance the sumoylation of the papillomavirus E1 protein. Virology. 2005;331:190–203.
Schmidt D, Muller S. PIAS/SUMO: new partners in transcriptional regulation. Cell Mol Life Sci. 2003;60:2561–74.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7.
Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–54.
Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–64.
Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29.
Ali MM, Yoshizawa T, Ishibashi O, Matsuda A, Ikegame M, Shimomura J, Mera H, Nakashima K, Kawashima H. PIASxbeta is a key regulator of osterix transcriptional activity and matrix mineralization in osteoblasts. J Cell Sci. 2007;120:2565–73.
Cooper SC, Flaitz CM, Johnston DA, Lee B, Hecht JT. A natural history of cleidocranial dysplasia. Am J Med Genet. 2001;104:1–6.
Yang Z, Griffith JF, Leung PC, Lee R. Effect of osteoporosis on morphology and mobility of the lumbar spine. Spine (Phila Pa 1976), 2009; 34:E115–21.
Reymond A, Meroni G, Fantozzi A, Merla G, Cairo S, Luzi L, Riganelli D, Zanaria E, Messali S, Cainarca S, Guffanti A, Minucci S, Pelicci PG, Ballabio A. The tripartite motif family identifies cell compartments. EMBO J. 2001;20:2140–51.
Miyajima N, Maruyama S, Bohgaki M, Kano S, Shigemura M, Shinohara N, Nonomura K, Hatakeyama S. TRIM68 regulates ligand-dependent transcription of androgen receptor in prostate cancer cells. Cancer Res. 2008;68:3486–94.
Vanderschueren D, Vandenput L, Boonen S, Lindberg MK, Bouillon R, Ohlsson C. Androgens and bone. Endocr Rev. 2004;25:389–425.
Huber DM, Bendixen AC, Pathrose P, Srivastava S, Dienger KM, Shevde NK, Pike JW. Androgens suppress osteoclast formation induced by RANKL and macrophage-colony stimulating factor. Endocrinology. 2001;142:3800–8.
Muñoz-Torres M, Jódar E, Quesada M, Escobar-Jiménez F. Bone mass in androgen-insensitivity syndrome: response to hormonal replacement therapy. Calcif Tissue Int. 1995;57:94–6.
Bertelloni S, Baroncelli GI, Federico G, Cappa M, Lala R, Saggese G. Altered bone mineral density in patients with complete androgen insensitivity syndrome. Horm Res. 1998;50:309–14.
Notini AJ, Davey RA, McManus JF, Bate KL, Zajac JD. Genomic actions of the androgen receptor are required for normal male sexual differentiation in a mouse model. J Mol Endocrinol. 2005;35:547–55.
Chiang C, Chiu M, Moore AJ, Anderson PH, Ghasem-Zadeh A, McManus JF, Ma C, Seeman E, Clemens TL, Morris HA, Zajac JD, Davey RA. Mineralization and bone resorption are regulated by the androgen receptor in male mice. J Bone Miner Res. 2009;24:621–3.
Acknowledgments
This work was supported by National Natural Science Foundation of China grants (no. 30772191).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Han, S., Zhu, Y., Wu, Z. et al. The differently expressed proteins in MSCs of degenerative scoliosis. J Orthop Sci 18, 885–892 (2013). https://doi.org/10.1007/s00776-013-0444-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00776-013-0444-8