Abstract
Background
Neuropathic pain is difficult to control and patient response to current treatment is often inadequate. Opioids have been widely used to treat a variety of pain states, but have several side effects. Endogenous opioids are clinically safe, but are not used for treatment because of rapid metabolism. However, in-vivo transfection of endogenous opioid genes could have a powerful and safe analgesic effect. The purpose of this study was to investigate the efficacy of proopiomelanocortin (POMC, a precursor of the endogenous opioid peptide β-endorphin) gene transfer by use of radial shock waves (RSWs) in a rat neuropathic pain model.
Methods
As a neuropathic pain model, we used the Bennett chronic constriction injury (CCI) method. Immediately after CCI induction, POMC plasmid was injected into the rats’ gastrocnemius muscle followed by exposure to RSW. Mechanical allodynia was measured for 4 weeks and dorsal root ganglion (DRG) neurons were sectioned and immunostained.
Results
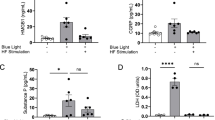
β-Endorphin blood levels and the number of β-endorphin-immunoreactive (IR) muscle fibers increased over 28 days. β-Endorphin overexpression caused a decrease in the number of calcitonin gene-related peptide (CGRP)-IR DRG neurons and suppressed neuropathic pain induced by CCI without causing adverse side effects. The size-distribution pattern of CGRP-IR DRG neurons shifted from small to large cells in the CCI group; however, the number of both small and large CGRP-IR cells decreased in the POMC group.
Conclusion
POMC gene transfection alleviated allodynia and reduced CGRP expression in DRG neurons without adverse effects. CGRP is not produced in large neurons under physiologic conditions; however, in this study CGRP expression was shifted to large neurons after nerve injury. This change in cell-size distribution suggests that CGRP expression in large neurons is related to neuropathic pain. These findings suggest that POMC gene transfection using RSWs is a safe and effective treatment for neuropathic pain.










Similar content being viewed by others
References
Attal N, Cruccu G, Baron R, Haanpää M, Hansson P, Jensen TS, Nurmikko T. European federation of neurological societies. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17:1113–23.
Russell K, Portenoy MD. Opioid therapy for chronic nonmalignant pain: a review of the critical issues. J Pain Symptom Manag. 1996;11:203–17.
Zuniga JR, Joseph SA, Knigge KM. The effect of nitrous oxide on the central endogenous pro-opiomelanocortin system in the rat. Brain Res. 1987;420:57–65.
Yaksh TL, Gross KE, Li CH. Studies on the intrathecal effect of beta-endorphin in primate. Brain Res. 1982;241:261–9.
Fink DJ, Wechuck J, Mata M, Glorioso JC, Goss J, Krisky D, Wolfe D. Gene therapy for pain: results of a phase I clinical trial. Ann Neurol. 2011;70:207–12.
Tatsis N, Ertl HC. Adenoviruses as vaccine vectors. Mol Ther. 2004;10:616–29.
Ritter T, Lehmann M, Volk HD. Improvement in gene therapy: averting the immune response to adenoviral vectors. BioDrugs. 2002;16:3–10.
Coonrod A, Li FQ, Horwitz M. On the mechanism of DNA transfection: efficient gene transfer without viruses. Gene Ther. 1997;4:1313–21.
Tachibana K, Uchida T, Ogawa K, Yamashita N, Tamura K. Induction of cell-membrane porosity by ultrasound. Lancet. 1999;353:1409.
Murata R, Nakagawa K, Ohtori S, Ochiai N, Arai M, Saisu T, Sasho T, Takahashi K, Moriya H. The effects of radial shock waves on gene transfer in rabbit chondrocytes in vitro. Osteoarthr Cartil. 2007;15:1275–82.
Saitoh Y, Taki T, Arita N, Ohnishi T, Hayakawa T. Cell therapy with encapsulated xenogeneic tumor cells secreting beta- endorphin for treatment of peripheral pain. Cell Transpl. 1995;1:S13–7.
Yamashita M, Yamauchi K, Suzuki M, Eguchi Y, Orita S, Endo M, Yamashita T, Takahashi K, Ohtori S. Transfection of rat cells with proopiomelanocortin gene, precursor of endogenous endorphin, using radial shock waves suppresses inflammatory pain. Spine. 2009;34:2270–7.
Bennett GJ, Xie Y. A peripheral mononeuropathy in rat that produces disorders of pin sensation like those seen in man. Pain. 1988;33:87–107.
Chuang IC, Jhao CM, Yang CH, Chang HC, Wang CW, Lu CY, Chang YJ, Lin SH, Huang PL, Yang LC. Intramuscular electroporation with the pro-opiomelanocortin gene in rat adjuvant arthritis. Arthritis Res Ther. 2004;6:R7–14.
Bennett DLH, Dmietrieva N, Priestley JV, et al. trkA, CGRP and IB4 expression in retrogradely labeled cutaneous and visceral primary sensory neurons in the rat. Neurosci Lett. 1996;206:33–6.
Lu CY, Chou AK, Wu CL, Yang CH, Chen JT, Wu PC, Lin SH, Muhammad R, Yang LC. Gene-gun particle with proopiomelanocortin cDNA produces analgesia against formalin-induced pain in rats. Gene Ther. 2002;9:1008–14.
Wood MJ, Charton HM, Wood KJ, Kajiwara K, Byrnes AP. Immune responses to adenovirus vectors in the nervous system. Trends Neurosci. 1996;19:497–501.
Mercadante S. Pain treatment and outcomes for patients with advanced cancer who receive follow-up care at home. Cancer. 1999;85:1849–58.
Miki K, Fukuoka T, Tokunaga A, Noguchi K. Calcitonin gene-related peptide increase in the rat spinal dorsal horn and dorsal column nucleus following peripheral nerve injury: upregulation in a subpopulation of primary afferent sensory neurons. Neuroscience. 1998;82:1243–52.
Ohtori S, Takahashi K, Moriya H. Calcitonin gene-related peptide immunoreactive sensory DRG neurons innervating the cervical facet joints in rats. J Orthop Sci. 2002;7:258–61.
Hökfelt T. Neuropeptides in perspective: the last ten years. Neuron. 1991;7:867–79.
Hosoya K, Ohtsuki S, Terasaki T. Recent advances in the brain-to-blood efflux transport across the blood-brain barrier. Int J Pharm. 2002;248:15–29.
Noguchi K, Kawai Y, Fukuoka T, Senba E, Miki K. Substance P induced by peripheral nerve injury in primary afferent sensory neurons and its effect on dorsal column nucleus neurons. J Neurosci. 1995;15:7633–43.
McCarthy PW, Lawson SN. Cell type and conduction velocity of rat primary sensory neurons with calcitonin gene-related peptide-like immunoreactivity. Neuroscience. 1990;34:623–32.
Wang R, Guo W, Ossipov MH, Vanderah TW, Porreca F, Lai J. Glial cell line-derived neurotrophic factor normalizes neurochemical changes in injured dorsal root ganglion neurons and prevents the expression of experimental neuropathic pain. Neuroscience. 2003;121:815–24.
Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–63.
Hammond DL, Ackerman L, Holdsworth R, Elzey B. Effects of spinal nerve ligation on immunohistochemically identified neurons in the L4 and L5 dorsal root ganglia of the rat. J Comp Neurol. 2004;475:575–89.
Liu WX, Wang R. Endomorphins: potential roles and therapeutic Indications in the development of opioid peptide analgesic drugs. Med Res Rev. 2011;32(3):536–80.
Jenks BG. Regulation of proopiomelanocortin gene expression: an overview of the signaling cascades, transcription factors, and responsive elements involved. Ann N Y Acad Sci. 2009;1163:17–30.
Acknowledgments
We did not receive grants or external funding in support of our research or preparation of this manuscript. We did not receive payments or other benefits or a commitment or agreement to provide such benefits from any commercial entity.
Author information
Authors and Affiliations
Corresponding author
Additional information
T. Ishikawa and M. Miyagi contributed equally to this work.
About this article
Cite this article
Ishikawa, T., Miyagi, M., Yamashita, M. et al. In-vivo transfection of the proopiomelanocortin gene, precursor of endogenous endorphin, by use of radial shock waves alleviates neuropathic pain. J Orthop Sci 18, 636–645 (2013). https://doi.org/10.1007/s00776-013-0397-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00776-013-0397-y