Abstract
Background
Orthopedic surgeons and dentists often implant materials to repair bone tissue defects and restore physiological functions of bone organs. The clinical success depends on adequate bone formation in operation sites. However, the real cause of osteogenesis has not yet been fully elucidated. To investigate the bone response to implanted materials, this study examined the bone tissue reaction in rat femoral medullary canal, which received gelatin and collagen as foreign-body materials.
Methods
A total of 36 six-month-old Sprague-Dawley rats were randomly and meanly divided into three groups. In the gelatin group, the bilateral femora received gelatin material; in the collagen group, they were implanted with type I collagen, and in the control group, the femora suffered from sham operation with no materials inserted. After 2, 4, 8, and 12 weeks, specimens were harvested and subjected to a series of examinations.
Results
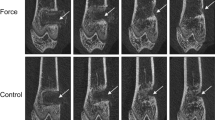
After 2 weeks of healing, a significant upregulation of both alkaline phosphatase and osteocalcin by both kinds of implanted materials relative to the control (sham implantation group) was seen in gene expression analysis. Strong reactivity of osteoprotegerin and receptor activator of NFκB ligand was detected in the two test groups in immunohistochemistry at 4 weeks of healing. Also, micro-CT revealed an increase in cortical bone thickness in the two test groups as compared to the control group. Densitometry showed increased bone mineral density in the bone receiving materials after 12 weeks, leading to the enhanced maximum load in the test groups.
Conclusions
These results indicated that the implanted materials led to an osteogenesis response in rat femoral medullary canal. Thus, we probably should reconsider the potential cascades of tissue reaction when utilizing orthopedic and dental implants and other materials to recover bone related-organ function and repair bone defects.






Similar content being viewed by others
References
Del Fattore A, Cappariello A, Teti A. Genetics, pathogenesis and complications of osteopetrosis. Bone. 2008;42:19–29.
Whyte MP, Wenkert D, McAlister WH, Novack DV, Nenninger AR, Zhang X, Huskey M, Mumm S. Dysosteosclerosis presents as an “osteoclast-poor” form of osteopetrosis: comprehensive investigation of a 3-year-old girl and literature review. J Bone Miner Res. 2010;25:2527–39.
Dennison E, Cole Z, Cooper C. Diagnosis and epidemiology of osteoporosis. Curr Opin Rheumatol. 2005;17:456–61.
Bab I, Gazit D, Massarawa A, Sela J. Removal of tibial marrow induces increased formation of bone and cartilage in rat mandibular condyle. Calcif Tissue Int. 1985;37:551–5.
Einhorn TA, Simon G, Devlin VJ, Warman J, Sidhu SP, Vigorita VJ. The osteogenic response to distant skeletal injury. J Bone Joint Surg Am. 1990;72:1374–8.
Ishizaka M, Tanizawa T, Sofue M, Dohmae Y, Endo N, Takahashi HE. Bone particles disturb new bone formation on the interface of the titanium implant after reaming of the marrow cavity. Bone. 1996;19:589–94.
Kondo N, Tokunaga K, Ito T, Arai K, Amizuka N, Minqi L, Kitahara H, Ito M, Naito M, Shu-Ying J, Oda K, Murai T, Takano R, Ogose A, Endo N. High dose glucocorticoid hampers bone formation and resorption after bone marrow ablation in rat. Microsc Res Tech. 2006;69:839–46.
Gazit D, Karmish M, Holzman L, Bab I. Regenerating marrow induces systemic increase in osteo- and chondrogenesis. Endocrinology. 1990;126:2607–13.
Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J. 2001;10(Suppl 2):S96–101.
Donath K, Laass M, Gunzl HJ. The histopathology of different foreign-body reactions in oral soft tissue and bone tissue. Virchows Arch A Pathol Anat Histopathol. 1992;420:131–7.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8.
Gungormus M, Kaya O. Evaluation of the effect of heterologous type I collagen on healing of bone defects. J Oral Maxillofac Surg. 2002;60:541–5.
Jegal SH, Park JH, Kim JH, Kim TH, Shin US, Kim TI, Kim HW. Functional composite nanofibers of poly(lactide-co-caprolactone) containing gelatin-apatite bone mimetic precipitate for bone regeneration. Acta Biomater. 2011;7:1609–17.
Young S, Wong M, Tabata Y, Mikos AG. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Control Release. 2005;109:256–74.
Tal H, Artzi Z, Moses O, Nemcovsky C, Kozlovsky A. Guided periodontal regeneration using bilayered collagen membranes and bovine bone mineral in fenestration defects in the canine. Int J Periodontics Restor Dent. 2005;25:509–18.
Llambes F, Silvestre FJ, Caffesse R. Vertical guided bone regeneration with bioabsorbable barriers. J Periodontol. 2007;78:2036–42.
Kim CS, Kim JI, Kim J, Choi SH, Chai JK, Kim CK, Cho KS. Ectopic bone formation associated with recombinant human bone morphogenetic proteins-2 using absorbable collagen sponge and beta tricalcium phosphate as carriers. Biomaterials. 2005;26:2501–7.
Gleeson JP, Plunkett NA, O’Brien FJ. Addition of hydroxyapatite improves stiffness, interconnectivity and osteogenic potential of a highly porous collagen-based scaffold for bone tissue regeneration. Eur Cell Mater. 2010;20:218–30.
Rothamel D, Schwarz F, Sager M, Herten M, Sculean A, Becker J. Biodegradation of differently cross-linked collagen membranes: an experimental study in the rat. Clin Oral Implants Res. 2005;16:369–78.
Bromme D, Okamoto K, Wang BB, Biroc S. Human cathepsin O2, a matrix protein-degrading cysteine protease expressed in osteoclasts. Functional expression of human cathepsin O2 in Spodoptera frugiperda and characterization of the enzyme. J Biol Chem. 1996;271:2126–32.
Gowen M, Lazner F, Dodds R, Kapadia R, Feild J, Tavaria M, Bertoncello I, Drake F, Zavarselk S, Tellis I, Hertzog P, Debouck C, Kola I. Cathepsin K knockout mice develop osteopetrosis due to a deficit in matrix degradation but not demineralization. J Bone Miner Res. 1999;14:1654–63.
Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–8.
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76.
Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res. 2000;15:2–12.
O’Brien CA. Control of RANKL gene expression. Bone. 2010;46:911–9.
Acknowledgments
This study was supported by a grant from the National Nature Science Foundation of China (No. 81070869).
Conflict of interest
The authors did not receive any benefits from any commercial party related directly or indirectly to this article, and there is no actual or potential conflict of interest among the authors in relation to this article.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Li, X., Feng, G., Zhu, S. et al. Osteogenesis response to implanted materials in endocortical bone in rat femora. J Orthop Sci 17, 626–633 (2012). https://doi.org/10.1007/s00776-012-0254-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00776-012-0254-4