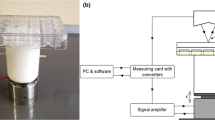
Abstract
Background
Low-magnitude vibration has been widely used as a tool for rehabilitation, enhancing physical performance, and stimulating bone development. Although mechanical stimulation generated by vibrations is regarded as important factor in bone remodeling, the underlying cellular and molecular regulatory mechanisms of this response, which may be important in the development of new mechanobiological strategies, currently remain unclear.
Methods
In this study, to investigate the mechanobiological mechanisms of vibration-enhanced osteogenic responses in osteoblasts, MC3T3-E1 cells were subjected to vibrations of different amplitude (0.06, 0.14, 0.32, 0.49, 0.66, and 0.8 × g) at 40 Hz for 30 min/day over 3 days. The osteogenesis-related transcription factors Wnt10B, Sclerostin, OPG, and RANKL were analyzed for mRNA and protein expression.
Results
The results revealed that protein expression of Wnt10B and OPG was increased in a magnitude-dependent manner by mechanical vibrations at amplitudes of 0.06, 0.14, 0.32, and 0.49 × g; the maximum increases were 2.4-fold (p < 0.001) and 7.9-fold (p < 0.001), respectively, at 0.49 × g. Sclerostin and RANKL levels were reduced at all amplitudes. On the basis of mRNA levels, the reduced expression of RANKL was further downregulated (p < 0.05) whereas OPG expression was further increased (p < 0.01) when the MC3T3-E1 cells were treated with LiCl compared with the effects of vibration alone.
Conclusions
The findings may indicate that Wnt signaling is involved in mechanotransduction at low-magnitude vibration; this may provide a cellular basis, and impetus for further development of, biomechanically based intervention for enhancing bone strength and accelerating implant osseointegration.






Similar content being viewed by others
References
Rubin C, Turner AS, Mallinckrodt C, Jerome C, McLeod K, Bain S. Mechanical strain, induced noninvasively in the high-frequency domain, is anabolic to cancellous bone, but not cortical bone. Bone. 2002;30:445–52.
Xie LQ, Jacobson JM, Choi ES, Busa B, Donahue LR, Miller LM, Rubin CT, Judex S. Low-level mechanical vibrations can influence bone resorption and bone formation in the growing skeleton. Bone. 2006;39:1059–66.
Leucht P, Kim JB, Wazen R, Currey JA, Nanci A, Brunski JB, Helms JA. Effect of mechanical stimuli on skeletal regeneration around implants. Bone. 2007;40:919–30.
Maloney WJ, Jasty M, Burke DW, O’Connor DO, Zalenski EB, Bragdon C, Harris WH. Biomechanical and histologic investigation of cemented total hip arthroplasties: a study of autopsyretrieved femurs after in vivo cycling. Clin Orthop Relat Res. 1989;249:129–40.
Goldring SR, Goldring MB. Eating bone or adding it: the Wnt pathway decides. Nat Med. 2007;13:133–4.
Lau E, Al-Dujaili S, Guenther A, Liu D, Wang L, You L. Effect of low-magnitude, high-frequency vibration on osteocytes in the regulation of osteoclasts. Bone. 2010;46:1508–15.
Stadelmann VA, Terrier A, Pioletti DP. Microstimulation at the bone–implant interface upregulates osteoclast activation pathways. Bone. 2008;42:358–64.
Midura RJ, Dillman CJ, Grabiner MD. Low amplitude, high-frequency strains imposed by electrically stimulated skeletal muscle retards the development of osteopenia in the tibiae of hindlimb suspended rats. Med Eng Phys. 2005;27:285–93.
Garman R, Gaudette G, Donahue LR, Rubin C, Judex S. Low-level accelerations applied in the absence of weight bearing can enhance trabecular bone formation. J Orthop Res. 2007;25:732–40.
Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, Macdougald OA. Regulation of osteoblastogenesis and bone mass byWnt10b. Proc Natl Acad Sci USA. 2005;102:3324–9.
Ellies DL, Viviano B, McCarthy J, Rey JP, Itasaki N, Saunders S, Krumlauf R. Bone density ligand, sclerostin, directly interacts with LRP5 but not LRP5G171V to modulate Wnt activity. J Bone Miner Res. 2006;21:1738–49.
Semenov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005;280:26770–5.
Brighton CT, Strafford B, Gross SB, Leatherwood DF, Williams JL, Pollack SR. The proliferative and synthetic response of isolated calvarial bone cells of rats to cyclic biaxial mechanical strain. J Bone Jt Surg. 1991;73:320–31.
Jones DB, Nolte H, Scholubbers JG, Turner E, Veltel D. Biochemical signal transduction of mechanical strain in osteoblastlike cells. Biomaterials. 1991;12:101–10.
Stanford CM, Morcuende JA, Brand RA. Proliferative and phenotypic responses of bone-like cells to mechanical deformation. J Orthop Res. 1995;13:664–70.
Tanaka SM, Li J, Duncan RL, Yokota H, Burr DB, Turner CH. Effects of broad-frequency vibration on cultured osteoblast. J Biomech. 2003;36:73–80.
Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2007;19:343–51.
Liu J, Zhao Z, Zou L, Li J, Wang F, Li X, Zhang J, Liu Y, Chen S, Zhi M, Wang J. Pressure-loaded MSCs during early osteodifferentiation promote osteoclastogenesis by increase of RANKL/OPG ratio. Ann Biomed Eng. 2009;37:794–802.
Rucci N, Rufo A, Alamanou M, Teti A. Modeled microgravity stimulates osteoclastogenesis and bone resorption by increasing osteoblast RANKL/OPG ratio. J Cell Biochem. 2007;100:464–73.
Kim CH, You L, Yellowley CE, Jacobs CR. Oscillatory fluid flow-induced shear stress decreases osteoclastogenesis through RANKL and OPG signaling. Bone. 2006;39:1043–7.
You L, Temiyasathit S, Lee P, Kim CH, Tummala P, Yao W, Kingery W, Malone AM, Kwon RY, Jacobs CR. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone. 2008;42:172–9.
Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter Babij P, Brown EL, Hill AA, Akhter MP, Johnson ML, Recker RR, Komm BS, Bex FJ. Wnt/β-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;42:31720–8.
Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektoret D, Jonas M, Kovacevich BR, Hampton KS, Appleby M, Brunkow ME, Latham JA. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22:6267–76.
Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, Feng G, Gao X, He L. Sclerostin mediates bone response to mechanical unloading via antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009;24:1651–61.
Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signaling in intact cells. Curr Biol. 1996;l6:1664–8.
Glass IIDA, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–64.
Ishigaki S, Nakano T, Yamada S, Nakamura T, Takashima F. Biomechanical stress in bone surrounding an implant under simulated chewing. Clin Oral Implants Res. 2003;12:97–102.
Lanyon L, Skerry T. Postmenopausal osteoporosis as a failure of bone’s adaptation to functional loading: a hypothesis. J Bone Miner Res. 2001;16:1937–47.
Collins JM, Ramamoorthy K, Da Silveira A, Patston P, Mao JJ. Expression of matrix metalloproteinase genes in the rat intramembranous bone during postnatal growth and upon mechanical stresses. J Biomech. 2005;38:485–92.
Qin YX, McLeod KJ, Guilak F, Rubin CT, Chiang FP. Correlation of bony ingrowth to the distribution of stress and strain parameters surrounding a porous-coated implant. J Orthop Res. 1996;14:862–8.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (no. 30772449).
Conflict of interest
The authors have no financial or personal relationships with other persons or organizations which may lead to a conflict of interest in this work.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hou, W.W., Zhu, Z.L., Zhou, Y. et al. Involvement of Wnt activation in the micromechanical vibration-enhanced osteogenic response of osteoblasts. J Orthop Sci 16, 598–605 (2011). https://doi.org/10.1007/s00776-011-0124-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00776-011-0124-5