Abstract
Background
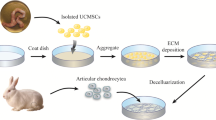
A scaffold-free cartilage construct, analogous to those found during embryonic precartilage condensation, has received much attention as a novel modality for tissue-engineered cartilage. In the present study, we developed an uncomplicated culture system by which scaffold-free cartilage-like tissues are produced using cell-cell interactions. With this system, we attempted to prevent dedifferentiation and reverse the phenotypic modulations by adjusting the cell density. We investigated whether low-intensity pulsed ultrasound (LIPUS) enhances matrix synthesis of the scaffold-free cartilage construct.
Methods
Rat articular chondrocytes multiplied in monolayers were seeded onto the synthetic porous membrane at stepwise cell densities (i.e., 1.0, 2.0, and 4.0 × 107 cells/cm2) to allow formation of a scaffold-free cartilage construct via cell-cell interaction. The cartilage constructs were then stimulated by LIPUS for 20 min/day. To investigate the effect of LIPUS stimulation on matrix synthesis, expression of mRNA for cartilage matrix molecules was quantified by a real-time reverse transcription-polymerase chain reaction. Synthesis of type II collagen, type I collagen, and proteoglycan was also assessed histologically.
Results
Only the chondrocytes cultured at high cell densities in the 2.0 × 107cells/cm2 group became concentrated and formed a plate-like construct similar to native articular cartilage by macroscopic and histological assessments. Statistical analysis on the matrix gene expression demonstrated that the levels of type II collagen and aggrecan mRNA of the 2.0 × 107cells/cm2 group were significantly higher than with the other two cell-density groups. Interestingly, the LIPUS application led to a statistically significant enhancement of aggrecan gene expression only in the 2.0 × 107 cells/cm2 group.
Conclusions
The current study presents a semi-open static culture system that facilitates production of the scaffold-free constructs from monolayer-cultured chondrocytes. It suggests that the LIPUS application enhances matrix production in the construct, and its combination with the scaffold-free construct might become a feasible tool for production of implantable constructs of better quality.
Similar content being viewed by others
References
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 1994;331:889–895.
Ruano-Ravina A, Jato Díaz M. Autologous chondrocyte implantation: a systematic review. Osteoarthritis Cartilage 2006;14:47–51.
Benya P, Shaffer J. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 1982;30:215–224.
Buschmann M, Gluzband Y, Grodzinsky A, Kimura J, Hunziker E. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J Orthop Res 1992;10:745–758.
Takahashi T, Ogasawara T, Asawa Y, Mori Y, Uchinuma E, Takato T, et al. Three-dimensional microenvironments retain chondrocyte phenotypes during proliferation culture. Tissue Eng 2007;13:1583–1592.
DeLise A, Fischer L, Tuan R. Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage 2000;8:309–334.
Mainil-Varlet P, Rieser F, Grogan S, Mueller W, Saager C, Jakob R. Articular cartilage repair using a tissue-engineered cartilage-like implant: an animal study. Osteoarthritis Cartilage 2001;9(suppl A):S6–15.
Marlovits S, Tichy B, Truppe M, Gruber D, Vécsei V. Chondrogenesis of aged human articular cartilage in a scaffold-free bioreactor. Tissue Eng 2003;9:1215–1226.
Park K, Huang J, Azar F, Jin R, Min B, Han D, et al. Scaffold-free, engineered porcine cartilage construct for cartilage defect repair: in vitro and in vivo study. Artif Organs 2006;30:586–596.
Nagai T, Furukawa K, Sato M, Ushida T, Mochida J. Characteristics of a scaffold-free articular chondrocyte plate grown in rotational culture. Tissue Eng Part A 2008;14:1183–1193.
Duarte LR. The stimulation of bone growth by ultrasound. Arch Orthop Trauma Surg 1983;101:153–159.
Heckman J, Ryaby J, McCabe J, Frey J, Kilcoyne R. Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J Bone Joint Surg Am 1994;76:26–34.
Kristiansen T, Ryaby J, McCabe J, Frey J, Roe L. Accelerated healing of distal radial fractures with the use of specific, lowintensity ultrasound: a multicenter, prospective, randomized, double-blind, placebo-controlled study. J Bone Joint Surg Am 1997;79:961–973.
Yang K, Parvizi J, Wang S, Lewallen D, Kinnick R, Greenleaf J, et al. Exposure to low-intensity ultrasound increases aggrecan gene expression in a rat femur fracture model. J Orthop Res 1996;14:802–809.
Parvizi J, Wu C, Lewallen D, Greenleaf J, Bolander M. Low-intensity ultrasound stimulates proteoglycan synthesis in rat chondrocytes by increasing aggrecan gene expression. J Orthop Res 1999;17:488–494.
Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta 1986;883:173–177.
Rahfoth B, Weisser J, Sternkopf F, Aigner T, von der Mark K, Bräuer R. Transplantation of allograft chondrocytes embedded in agarose gel into cartilage defects of rabbits. Osteoarthritis Cartilage 1998;6:50–65.
Homminga G, Buma P, Koot H, van der Kraan P, van den Berg W. Chondrocyte behavior in fibrin glue in vitro. Acta Orthop Scand 1993;64:441–445.
Guo J, Jourdian G, MacCallum D. Culture and growth characteristics of chondrocytes encapsulated in alginate beads. Connect Tissue Res 1989;19:277–297.
Yasui N, Osawa S, Ochi T, Nakashima H, Ono K. Primary culture of chondrocytes embedded in collagen gels. Exp Cell Biol 1982;50:92–100.
Wakitani S, Kimura T, Hirooka A, Ochi T, Yoneda M, Yasui N, et al. Repair of rabbit articular surfaces with allograft chondrocytes embedded in collagen gel. J Bone Joint Surg Br 1989;71:74–80.
Solchaga L, Yoo J, Lundberg M, Dennis J, Huibregtse B, Goldberg V, et al. Hyaluronan-based polymers in the treatment of osteochondral defects. J Orthop Res 2000;18:773–780.
Funayama A, Niki Y, Matsumoto H, Maeno S, Yatabe T, Morioka H, et al. Repair of full-thickness articular cartilage defects using injectable type II collagen gel embedded with cultured chondrocytes in a rabbit model. J Orthop Sci 2008;13:225–232.
Watt F. Effect of seeding density on stability of the differentiated phenotype of pig articular chondrocytes in culture. J Cell Sci 1988;89(Pt 3):373–378.
Brehm W, Aklin B, Yamashita T, Rieser F, Trüb T, Jakob R, et al. Repair of superficial osteochondral defects with an autologous scaffold-free cartilage construct in a caprine model: implantation method and short-term results. Osteoarthritis Cartilage 2006;14:1214–1226.
Stoddart M, Ettinger L, Häuselmann H. Generation of a scaffold free cartilage-like implant from a small amount of starting material. J Cell Mol Med 2006;10:480–492.
Takahashi I, Nuckolls G, Takahashi K, Tanaka O, Semba I, Dashner R, et al. Compressive force promotes SOX9, type II collagen and aggrecan and inhibits IL-1 beta expression resulting in chondrogenesis in mouse embryonic limb bud mesenchymal cells. J Cell Sci 1998;111(Pt 14):2067–2076.
Elder S, Kimura J, Soslowsky L, Lavagnino M, Goldstein S. Effect of compressive loading on chondrocyte differentiation in agarose cultures of chick limb-bud cells. J Orthop Res 2000;18:78–86.
Grogan SP, Rieser F, Winkelmann V, Berardi S, Mainil-Varlet P. A static, closed and scaffold-free bioreactor system that permits chondrogenesis in vitro. Osteoarthritis Cartilage 2003;11:403–411.
Cao L, Lee V, Adams M, Kiani C, Zhang Y, Hu W, et al. Betaintegrin-collagen interaction reduces chondrocyte apoptosis. Matrix Biol 1999;18:343–355.
Jin RL, Park SR, Choi BH, Min BH. Scaffold-free cartilage fabrication system using passaged porcine chondrocytes and basic fibroblast growth factor. Tissue Eng Part A 2009;15:1887–1895.
Pacifici M, Golden EB, Adams SL, Shapiro IM. Cell hypertrophy and type X collagen synthesis in cultured articular chondrocytes. Exp Cell Res 1991;192:266–270.
Nishikori T, Ochi M, Uchio Y, Maniwa S, Kataoka H, Kawasaki K, et al. Effects of low-intensity pulsed ultrasound on proliferation and chondroitin sulfate synthesis of cultured chondrocytes embedded in atelocollagen gel. J Biomed Mater Res 2002;59:201–206.
Mauck R, Seyhan S, Ateshian G, Hung C. Influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte-seeded agarose hydrogels. Ann Biomed Eng 2002;30:1046–1056.
Author information
Authors and Affiliations
About this article
Cite this article
Uenaka, K., Imai, S., Ando, K. et al. Relation of low-intensity pulsed ultrasound to the cell density of scaffold-free cartilage in a high-density static semi-open culture system. J Orthop Sci 15, 816–824 (2010). https://doi.org/10.1007/s00776-010-1544-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00776-010-1544-3