Abstract
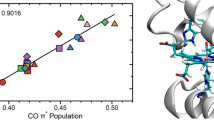
Quantum chemical geometry optimisations have been performed on realistic models of the active site of myoglobin using density functional methods. The energy of the hydrogen bond between the distal histidine residue and CO or O2 has been estimated to be 8 kJ/mol and 32 kJ/mol, respectively. This 24 kJ/mol energy difference accounts for most of the discrimination between CO and O2 by myoglobin (about 17 kJ/mol). Thus, steric effects seem to be of minor importance for this discrimination. The Fe—C and C—O vibrational frequencies of CO-myoglobin have also been studied and the results indicate that CO forms hydrogen bonds to either the distal histidine residue or a water molecule during normal conditions. We have made several attempts to optimise structures with the deprotonated nitrogen atom of histidine directed towards CO. However, all such structures lead to unfavourable interactions between the histidine and CO, and to νCO frequencies higher than those observed experimentally.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 7 July 1998 / Accepted: 26 October 1998
Rights and permissions
About this article
Cite this article
Sigfridsson, E., Ryde, U. On the significance of hydrogen bonds for the discrimination between CO and O2 by myoglobin. JBIC 4, 99–110 (1999). https://doi.org/10.1007/s007750050293
Issue Date:
DOI: https://doi.org/10.1007/s007750050293