Abstract
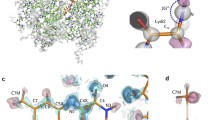
The structure of desulfoferrodoxin (DFX), a protein containing two mononuclear non-heme iron centres, has been solved by the MAD method using phases determined at 2.8 Å resolution. The iron atoms in the native protein were used as the anomalous scatterers. The model was built from an electron density map obtained after density modification and refined against data collected at 1.9 Å. Desulfoferrodoxin is a homodimer which can be described in terms of two domains, each with two crystallographically equivalent non-heme mononuclear iron centres. Domain I is similar to desulforedoxin with distorted rubredoxin-type centres, and domain II has iron centres with square pyramidal coordination to four nitrogens from histidines as the equatorial ligands and one sulfur from a cysteine as the axial ligand. Domain I in DFX shows a remarkable structural fit with the DX homodimer. Furthermore, three β-sheets extending from one monomer to another in DFX, two in domain I and one in domain II, strongly support the assumption of DFX as a functional dimer. A calcium ion, indispensable in the crystallisation process, was assumed at the dimer interface and appears to contribute to dimer stabilisation. The C-terminal domain in the monomer has a topology fold similar to that of fibronectin III.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 16 April 1997 / Accepted: 31 July 1997
Rights and permissions
About this article
Cite this article
Coelho, A., Matias, P., Fülöp, V. et al. Desulfoferrodoxin structure determined by MAD phasing and refinement to 1.9-Å resolution reveals a unique combination of a tetrahedral FeS4 centre with a square pyramidal FeSN4 centre. JBIC 2, 680–689 (1997). https://doi.org/10.1007/s007750050184
Issue Date:
DOI: https://doi.org/10.1007/s007750050184